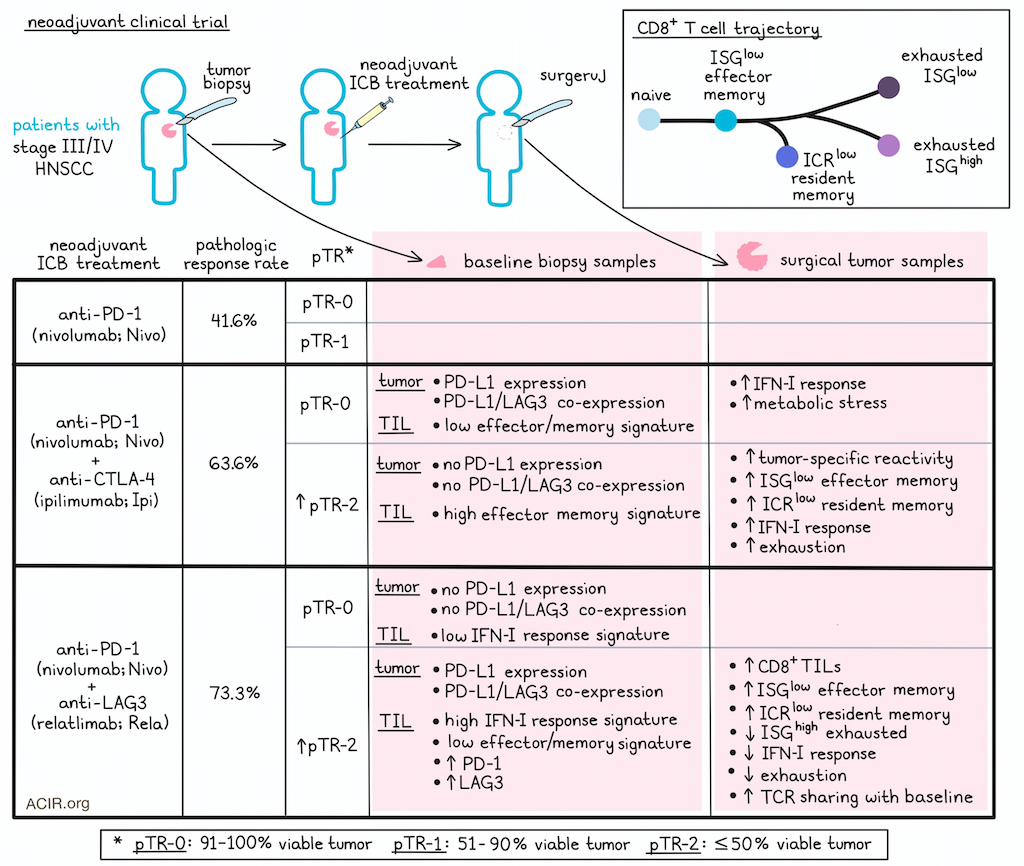
Treatment with checkpoint blockades alone or in various combinations can induce strong antitumor responses, but identifying which treatment or combination will work best for each individual patient is still a challenge. In work recently published in Cancer Cell, Li and Zandberg et al. evaluated results and T cell dynamics from a neoadjuvant trial of nivolumab (Nivo; anti-PD-1) alone or in combination with ipilimumab (Ipi; anti-CTLA-4) or relatlimab (Rela; anti-LAG3) in patients with stage III-IVa head and neck squamous cell carcinoma (HNSCC). This allowed insights into mechanisms of action of each combination, and the identification of biomarkers of response in the different therapy arms.
Looking first at the clinical outcomes in this trial, Li and Zandberg et al. categorized 38 evaluable patients into 3 groups based on their pathologic tumor response (pTR): pTR-0 had 91–100% viable residual tumor, pTR-1 had 51%–90% viable residual tumor, and pTR-2 had 50% or less viable residual tumor. Both of the combination treatment arms had higher pathologic response rates (73.3% Nivo+Rela, 63.6% Nivo+Ipi) than Nivo alone (41.6%), and saw higher portions of patients in the pTR-2 groups. In the Nivo+Ipi group, tumors without expression of PD-L1 or PD-L1 and LAG3 had higher pathologic responses, while in the Nivo+Rela group, tumors with PD-L1 and LAG3 co-expression had higher pathologic responses. At a median follow-up of 32.2 months, overall survival (OS) and disease-free survival (DFS) were comparable between the arms, but patients in pTR-2 groups trended towards better OS and DFS.
To study whether the transcriptional profiles of T cells were related to pathologic responses in neoadjuvant checkpoint blockade, TILs were isolated from 35 patients, with 20 patients having matched pre- and post-treatment biopsies. Across arms, increased CD8+ TILs at baseline trended towards a correlation with better pathologic response, while the expression of PDCD1 and LAG3 by baseline CD8+ TILs positively correlated with the degree of pathologic response only in Nivo+Rela-treated patients. Evaluating differentially expressed genes and gene set enrichment between pTR-2 and pTR-0 groups for each regimen, the researchers found that in the Nivo+Ipi arm, genes upregulated in pTR-2 patients were associated with TCR signaling, inflammation, effector function, memory, and tissue residency, while in the Nivo+Rela arm, genes upregulated inTR-2 patients were associated with IFN-I response/stimulation, inhibitory receptors, transcription factors associated with T cell exhaustion, HLA-II expression, homing, and an effector T cell state. In two patients with progressive disease who were not evaluable for pTR, baseline CD8+ TILs were less activated and had elevated expression of naive/central memory T cell-associated markers.
Looking at CD8+ TIL densities within tumors, the researchers found that only Nivo+Rela-treated pTR-2 patients showed a statistically significant increase in CD8+ TIL abundance on treatment, and these patients also showed the most prominent increases in CD8+ T cell density across both the whole tumor and the outer tumor-invasive margins, with this increase being associated with pathologic response.
Next, CD8+ TILs were isolated and grouped into 12 transcriptionally defined subclusters, and changes in CD8+ TIL activation states were assessed for paired baseline and post-treatment tissues. This revealed that in pTR-2 patients treated with Nivo+Rela, there was a significant increase in ISGlow effector memory-like T cells and a decrease in ISGhigh exhausted-like T cells. As ISGlow effector memory-like T cells were the most abundant phenotype in both Nivo+Rela and Nivo+Ipi-treated pTR-2 patients, the researchers compared these cells between pTR-2 and pTR-0 patients and found that in the Nivo+Ipi pTR-2 cohort, effector phenotypes and signs of tumor-specific reactivity were enhanced compared to pTR-0, which showed signs of IFN-I response and metabolic stress. Further, many upregulated genes were shared between ISGlow effector memory-like T cells in the Nivo+Ipi and Nivo+Rela pTR-2 patients and in comparisons of pTR-0 and pTR-2 groups between arms. Similar results were observed in an immune checkpoint receptor (ICR)low resident-memory-like subset. These results suggested that T cells in tumors responding to both combination treatments showed similar effector T cell profiles post-treatment, despite varying baseline CD8+ TIL states.
Evaluation of T cell clonal dynamics showed that the increase of ISGlow effector memory-like T cells and the ICRlow resident-memory-like subset was likely due to the expansion of pre-existing clonotypes in tumors, regardless of response or therapeutic regimen, though TCR sharing between baseline and post-treatment tumors was greatest in the Nivo+Rela pTR-2 cohort. In Nivo+Ipi-treated pTR-2 patients, a majority of pre-existing CD8+ TILs already displayed an effector memory phenotype prior to treatment, whereas only a small portion of pre-existing cells exhibited the same phenotype at baseline in pTR-2 Nivo+Rela-treated patients. Based on TCR sharing between various transcriptional states at baseline versus post-treatment in the different treatment arms, the researchers reconstructed differentiation trajectories in which naive/memory T cells transitioned into ISGlow effector memory T cells, which then either branched off early into resident memory ICRlow cells or later into either exhausted ISGhigh or exhausted ISGlow phenotypes. In the Nivo+Rela pTR-2 cohort, this trajectory was shifted towards earlier differentiation states, while in the Nivo+Ipi pTR-2 cohort, baseline samples were enriched for cells at earlier points in this trajectory, then shifted more towards the resident memory path after therapy.
Investigating the mechanisms underlying these differences, Li and Zandberg et al. identified tumor-reactive TILs based on co-expression of CD103, CD39, and CXCL13, and found that they were enriched for ISGhigh exhausted and ICRlow resident memory phenotypes. Interrogation of changes in tumor-reactive CD8+ TIL from baseline to after treatment showed that T cells from Nivo+Rela-treated pTR-2 patients demonstrated reduced expression of genes associated with exhaustion and IFN-I response compared to pTR-0. In contrast, T cells from Nivo+Ipi pTR-2 patients showed increased expression of IFN-I and exhaustion gene programs.
Overall, these results show that patients with HNSCC show improved pathologic responses with combination therapies over monotherapy. Pathologic responders to Nivo+Ipi or Nivo+Rela showed distinct CD8+ TIL profiles at baseline, which could help to stratify patients for treatment selection. On-treatment T cell evaluation showed that Nivo+Rela works by targeting and reprogramming exhausted CD8+ TILs with IFN-I-stimulated gene signatures, promoting their transition to an effector profile, and resulting in increased TCR diversity and widespread TCR sharing among different transcriptional states in pathologic responders. Meanwhile, Nivo+Ipi works mainly by activating and expanding pre-existing effector memory and resident memory TILs.
Write-up and image by Lauren Hitchings




