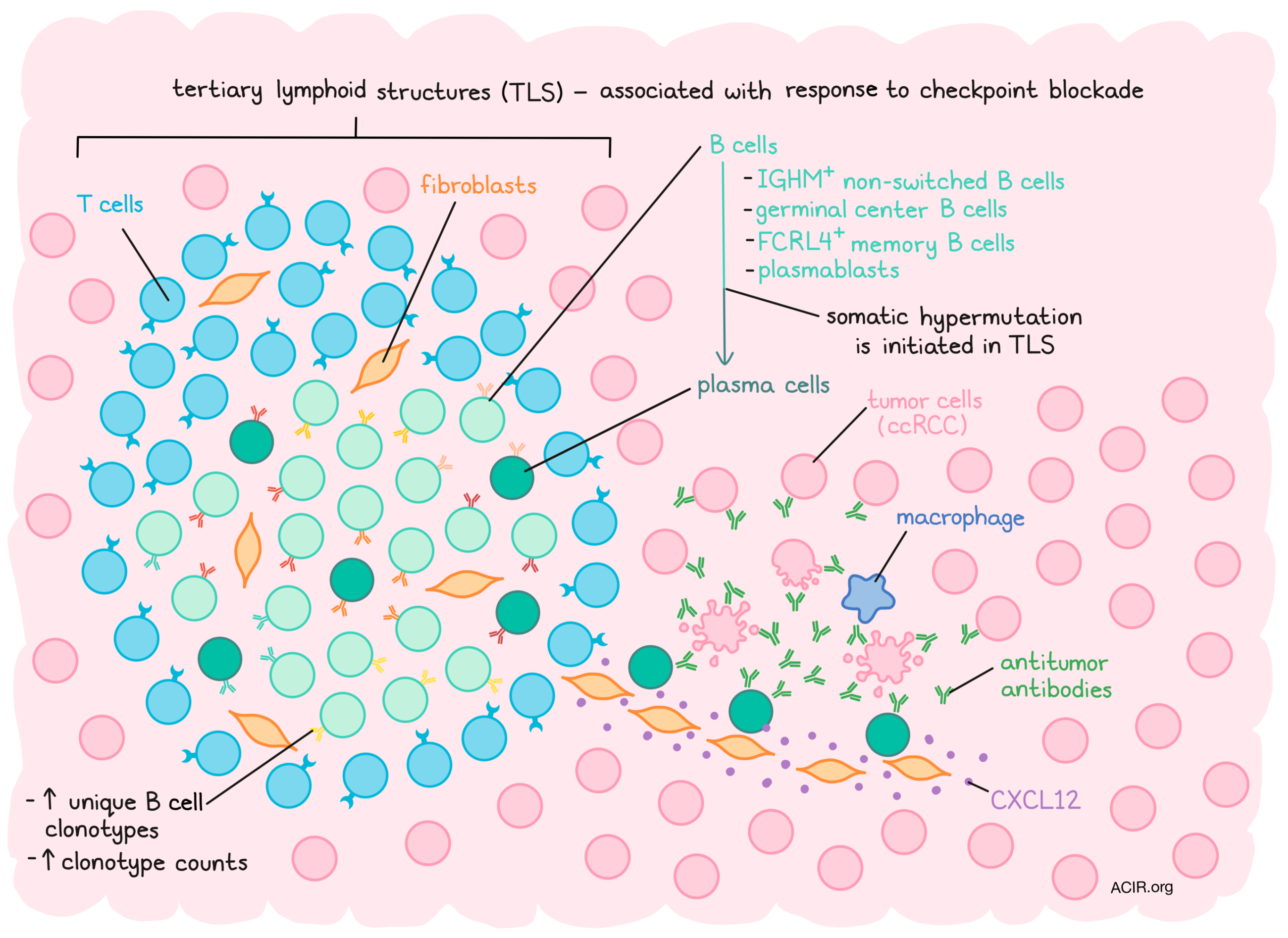
While tertiary lymphoid structures (TLS), organized lymphoid structures found in sites of chronic inflammation and antigen exposure, have been implicated in improved outcome and response to immunotherapy in cancer, the role of B cell responses associated with these structures is not well known. Therefore, Meylan et al. studied the role of TLS on B cell responses in the tumor microenvironment (TME) of clear cell renal cell carcinoma (ccRCC) using spatial transcriptomics, RNAseq, and multicolor histochemical and fluorescent immunostaining. Their results were recently published in Immunity.
Tumor samples from three ccRCC cohorts were used for this study, 24 tumors were characterized by Visium spatial transcriptomics technology (an arrayed, bar-coded, bead-based approach that allows transcriptome analysis across a tissue section), and 130 tumors were analyzed using spatial imaging using IHC and IF, as well as an artificial intelligence (AI)-based proximity assessment between cells. Using MCP-counter to quantify cell types, the researchers assessed the immune and stromal cell type abundance from the transcriptome data. In tumors with TLS, B lineage and T cell gene signatures mainly were concentrated in TLS areas, along with a high expression of the fibroblast signature. Tumors without TLS, on the other hand, did not show such patterns, with low and diffusely distributed B lineage and other immune scores.
Differential gene expression analyses between TLS and tumor areas identified a 29-gene TLS imprint signature that included 12 immunoglobulin (Ig) genes, 5 B cell markers, 2 T cell markers, 2 fibroblast markers, and 2 complement protein-coding genes and 6 other markers. Because Ig and B cell-associated genes dominated this signature, further analyses focused on B cells. The authors used robust cell type decomposition to map the differentiation state of B cell subsets spatially within the TME. TLS had limited expression of naive B cell-associated genes There was a strong correlation between TLS and FCRL4+ memory B cell, germinal center (GC) B cell, and plasmablast signatures. While expression was generally low in the TME, the TLS expressed high levels of the plasma cell (PC)-associated gene MZB1. Importantly, this gene was also found dispersed over the tumor area, even at a distance from the TLS. Since these cells produce antibodies, the spatial expression of Ig genes was also assessed. IGHM was mainly restricted to TLS, indicating the presence of non-switched B cells, while IGHG1, IGHA1, and pan Ig genes were highly expressed in TLS and away from the TLS in the tumor.
The location of the Ig genes was associated with high expression of the pan fibroblast marker COL1A1, along with other fibroblast signatures. The colocalization of the TLS signature with MZB1, fibroblasts, and CXCL12 – a chemokine associated with PC migration - was confirmed, as well as colocalization of fibroblasts, CXCL12, and MZB1 at a distance from the TLS. These correlations were only found in tumors that contained TLS. These data suggest that in tumors with TLS, IGHG1 and IGHA1-producing PCs are present in the TLS area and may infiltrate the tumor tissue in association with fibroblasts.
During B cell adaptive immunity generation, B cells undergo mutation of the B cell receptor (BCR) sequence to select the most affine clones. To study this, the researchers performed spatial BCR repertoire profiling. Tumors with TLS had the highest number of unique clonotypes and total Ig counts. The full range of mutations (low to high) was detected in TLS areas, while the Ig found in tumor areas were enriched in highly mutated sequences. These data suggest that somatic hypermutation is initiated in the TLS and that mutated PCs then infiltrate into the tumor tissue. Studying the heavy chain (IgH) repertoire, Meylan et al. found that high TLS score samples had a high number of clonotype counts, with 30% of the repertoire being identified over 100 times, which was only 1% for tumors low in TLS. In addition, clonotypes of the same family were found clustered close to the TLS and sparsely disseminated at a distance in the tumor, with some found at up to 3 mm from the TLS.
To confirm the above data, the location of PCs, fibroblasts, and expression of CXCL12 were assessed using multiplex IF of tumor sections. IgG+ and IgA+ MUM1+ PCs were detected in the TLS areas, with CXCL12+ COL1+ fibroblasts in the same location. PCs were also detected at a distance from the TLS in a network of fibroblasts, with most PCs being very close to fibroblast nuclei. The presence of these PCs was also strongly associated with TLS maturation markers.
The researchers then used labeled anti-human Ig antibodies to investigate whether the Ig in tumor tissue represented antitumor antibodies. Tumors with TLS showed a high percentage of anti-IgG labeling, consistent with endogenous antibody decoration of tumor cells, with weak anti-IgA labeling in both TLS and non-TLS tumors. The labeling of IgG of tumor cells was correlated with IgG+ PC infiltration. To determine whether these antibodies conveyed an antitumor effect, apoptosis of the tumor cells was assessed. The density of cleaved caspase 3+ tumor cells was higher in tumors with an IgG staining of over 60% (the optimal discriminatory cut-off). There was no correlation with the overall density of macrophages and NK cells in these tumors; however, tumors with high numbers of apoptotic cells and a high percentage of IgG+ tumor cells were more infiltrated with macrophages, which were located near apoptotic tumor cells.
Finally, Meylan et al. assessed the influence of IgG tumoral staining on the clinical response to immune checkpoint blockade (ICB). In pre-treatment samples from patients treated with nivolumab and ipilimumab, there was a correlation between clinical responses (62% objective response [OR]) for those who had tumors with IgG staining above the discriminatory mean, while those with lower tumor labeling had an OR of 18%. Patients with a high level of labeling also had prolonged progression-free survival.
These data suggest an essential role of TLS in initiating tumor-specific B cell responses and the presence of tumor-specific antibodies, perhaps originating from the induced plasma cells, that may improve response to ICB. Therefore, inducing TLS or making use of the locally produced antibodies may be new therapeutic avenues to explore.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author Maxime Meylan answered our questions.

What prompted you to tackle this research question?
In 2020, it was demonstrated that the presence B cells and their aggregation in tertiary lymphoid structures (TLS) have an impact on the response to immunotherapy in cancer. However, the biological functions of B cells and TLS were unknown. To decipher the roles of B cells and TLS, we needed to understand the spatial context of the tumor microenvironment. Therefore, we used spatial transcriptomics and multiplex immunofluorescence to analyze tumors bearing such structures.
What was the most surprising finding of this study for you?
The main result featured in the study is actually an accidental discovery! We were drawing concentric circles around the TLS to evidence gradients in gene expression, notably in cytokines. The most varying genes were immunoglobulins (Ig), which were expected to be high in the TLS and low in the tumor. We confirmed the Ig levels were high in the TLS, and low in the TLS periphery; however, as we investigated further in the tumor area, Ig levels went high again, and at similar levels to in TLS! This was the beginning of the story of how plasma cells are produced in the TLS and disseminated in the tumor area to produce antitumor antibodies.
What was the coolest thing you’ve learned (about) recently outside of the lab?
I recently moved to the US to start a postdoc. Now that I have completely moved in, I can proudly affirm that I am an expert in assembling Ikea furniture!




