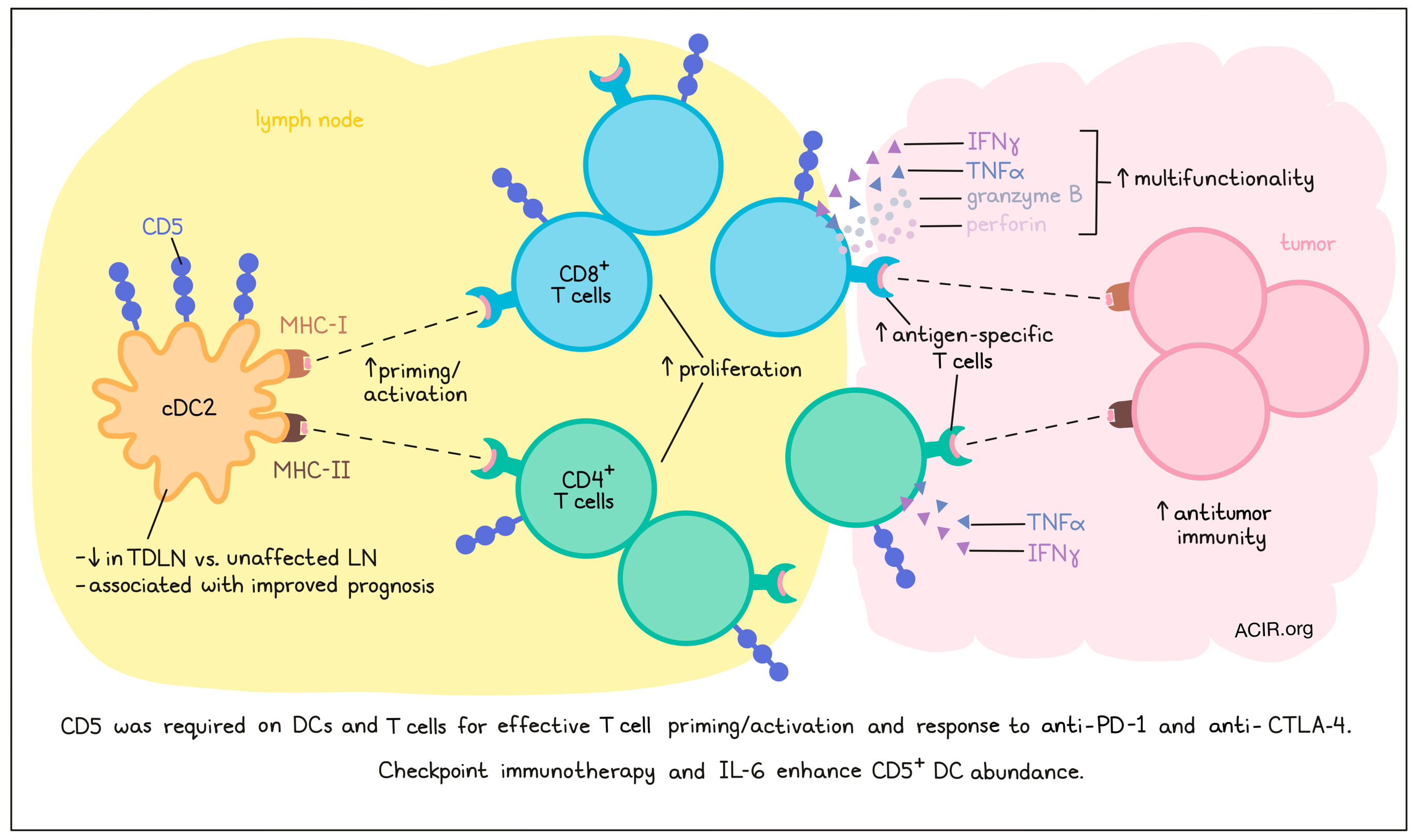
To induce effective antitumor T cell responses in response to immune checkpoint blockade (ICB) treatment, effective priming by dendritic cells (DCs) is essential. However, the characteristics of effective DC responses during ICB remain largely unclear. Having previously shown that migratory CD5+ DCs in the skin can prime CD4+ and CD8+ T cells more effectively than other DC subsets, He et al. assessed the role of this DC subset in antitumor immunity and ICB therapy responses. Their results were recently published in Science.
The researchers sorted myeloid cells from tumor-draining lymph nodes (TDLNs) and unaffected lymph nodes of patients with metastatic melanoma, and assessed these cells by single-cell RNAseq. CD5 expression was mostly detected on cDC2s in the unaffected LNs. Confirming these data, flow cytometry and CyTOF analysis confirmed the presence of CD1c+CD5+ DCs in uninvolved LNs. Assessing the prognostic role of CD5 using TCGA data showed that CD5 expression and the CD5+ DC signature were associated with improved prognosis in melanoma, lung squamous cell carcinoma, sarcoma, breast cancer, cervical squamous cell carcinoma, and endocervical adenocarcinoma.
In vitro experiments showed that CD5+ DCs had enhanced capacity to activate and induce proliferation of naive CD4+ and CD8+ T cells, resulting in higher production of IFNγ and TNFα by the T cells. To determine whether CD1c+CD5+ DCs were capable of reactivating CD8+ T cells specific to influenza matrix protein M1 (Flu-M1), DCs were isolated from an HLA-A2+ donor, and loaded with a peptide containing the HLA-A2-restricted epitope from Flu-M1. Coculturing these DCs with autologous peripheral CD8+ T cells resulted in higher antigen-specific T cell frequencies than coculture with other DC populations. Similarly, CD5+ DCs more efficiently activated MHC-II-restricted viral CD4+ T cell responses.
To determine the impact of CD5 expression levels, dermal CD1c+ DCs were sorted based on the number of CD5 molecules. Coculture experiments showed that the most effective priming of CD8+ T cells was induced by CD5hi DCs, resulting in a higher frequency of CD8+ T cells producing IFNγ, granzyme B, and perforin. CD5hi DCs also primed a higher frequency of multifunctional T cells expressing two or three effector molecules than CD5int or CD5- DCs. CD5hi DCs also induced higher proliferation of CD4+ T cells and IFNγ production than the other DC subsets. These effects were inhibited when a blocking CD5 antibody was added to the culture. On the other hand, when CD5 was upregulated on CD5- DCs by CRISPR gene activation, naive T cell proliferation and priming increased, suggesting that CD5 expression on DCs plays an essential role in these processes.
In contrast to the human data, CD5 was found on both cDC1 and cDC2 subsets in mice. The researchers generated conditional CD5 knockout mice using CRISPR-Cas9 to delete CD5 specifically in DCs (CD5ΔDC). To determine whether CD5 expression on DCs was required to induce antitumor immune responses in vivo, the MCA-induced syngeneic sarcoma cell line engineered to express ovalbumin (OVA) (MCA1956-mOVA) was used. While 14/15 WT mice eliminated tumors, only 2/11 CD5ΔDC mice rejected the tumor, and tumor-reactive CD8+ T cells expanded only in control mice.
To assess the implications on CD4+ T cell responses, antigen-specific T cell activation assays were performed using co-culturing of CD5-deficient DCs or control cDC2s with purified T cells from OT-II mice in the presence of a long peptide containing the MHC-II-restricted epitope. This showed that the CD5- DCs were less efficient at activating and inducing the proliferation of OT-II T cells.
Next, the researchers assessed the effects of CD5 expression on DCs for the efficacy of ICB therapy. Parental MCA1956 tumors grew progressively in control and CD5ΔDC mice, but only control mice responded to anti-PD-1 treatment. Similar results were obtained for anti-CTLA-4 therapy. Using the MC38 colorectal cancer model, the researchers found that the frequency of CD8+ T cells reactive to mutated epitopes from the mutated ADP-specific glucokinase (a neoantigen in MC38) was lower in tumors of CD5ΔDC mice, suggesting reduced priming.
In mice with CD5-deficient T cells (CD5ΔT), the growth of MCA1956 tumors was comparable with control mice. However, most of the control mice (13/16) responded to anti-PD-1 treatment, while none of the 14 CD5ΔT mice rejected the tumor. Similarly, in mice with CD5-deficient T cells, MCA 1956-mOVA tumors were not spontaneously rejected, and these mice had reduced numbers of OVA-specific CD8+ T cells.
ICB treatment in control mice did not alter the frequencies of DCs and macrophages, but it did upregulate the frequency of CD5+ DCs in the tumor and TDLN. CD5+ DCs were mainly found in the LN after treatment, and had lower levels of CCR7, suggesting the increase was not due to increased migration of these DCs. However, in vitro experiments showed that CD5+ DCs were more resistant to apoptosis than CD5- DCs in the presence of anti-PD-1.
In human T cells isolated from LNs, a larger fraction of T cells was found to express CD5 in LNs unaffected by tumor than in corresponding metastatic tumor LNs. In vitro stimulation of primary human T cells in which CD5 was deleted using CRISPR-Cas9 showed that CD5 expression was essential for T cell activation.
To assess what factors might impact CD5+ DCs presence in response to anti-PD-1 in humans, the researchers looked at the DCs from a patient with inherited PD-1 deficiency. This patient had higher frequencies of CD5+ DCs than controls. This led the researchers to hypothesize a role for IL-6, which is excessively produced in this deficiency. In mice treated with anti-PD-1, higher levels of IL-6 were detected in the tumor, and in vitro experiments showed that the presence of IL-6 during DC differentiation resulted in more CD5+ DCs. High concentrations of IL-6 skewed the development from DC1 toward DC2, while low IL-6 concentrations resulted in increased CD5 on both cDC1 and cDC2.
In conclusion, the data presented here suggest an essential role of CD5+ DCs in antitumor responses, in particular, in response to ICB. Treatments directed at increasing the number of CD5+ DCs may therefore be an attractive approach to increase the response to ICB in patients who do not benefit from this therapy.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, lead author Eynav Klechevsky answered our questions.

What was the most surprising finding of this study for you?
We were surprised to find that CD5 was expressed on mouse DCs. We already knew that it was on human DCs, and finding it in the mouse as well enabled us to develop a mouse model to examine its role in vivo in a preclinical model of disease. By studying the expression and function of CD5 in both species, we gain valuable insights into the role of this protein in the immune system, and how we can leverage this to accelerate the design of more effective treatments for a range of diseases and conditions.
What is the outlook?
As our next step, we aim to investigate how the abundance of CD5+ DCs can serve as a biomarker not only for T cell effector quality, but also for responsiveness to immunotherapy. We aim to understand how the CD5 on DCs facilitate communication with T cells, antigen handling, and directing specific types of T cells, particularly memory T cells, which are crucial in preventing tumor recurrence in patients. In addition, we aim to develop methods to expand these cells in patients and activate them effectively in the context of novel immunotherapy approaches.
What was the coolest thing you’ve learned (about) recently outside of work?
As a mother and a scientist, I learned that our children get inspired by what we do in the lab. Like me, they are curious about how life works and are enjoying exploring their interests to make exciting discoveries.




