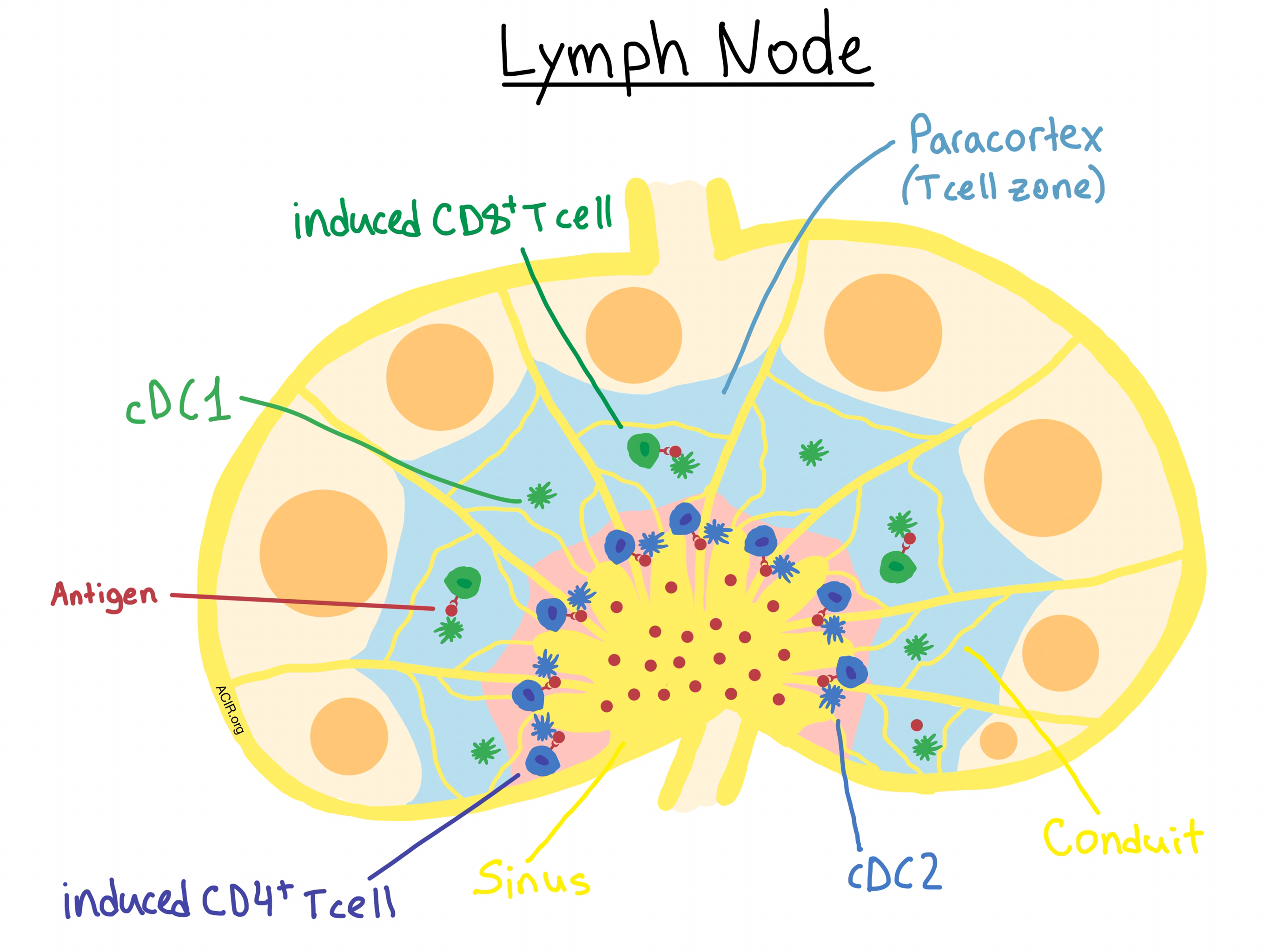
Form follows function, and the fascinating structural microanatomy and related immunological functioning of lymph nodes revealed by Gerner et al. in The Journal of Experimental Medicine highlight their importance for rational vaccine design. Dendritic cells (DCs) within the lymphatic tissues are the key initiators of adaptive immune responses during immunization or in the presence of a growing tumor, but their subsets are known to be asymmetrically distributed within the lymph node (LN). Using several quantitative imaging techniques, including two-photon intravital imaging, multiplex confocal microscopy with 3D reconstruction, and multiplex immunohistochemistry, Gerner et al. explored how antigen dispersal within the LN and the uptake and presentation of antigens by asymmetrically distributed DCs affect the activation of naive T cells after protein immunization.
Two protein antigen models were used for visualization and immunization: EαGFP to image antigen draining, uptake, and presentation by MHC II molecules, and OVA to allow direct assessment of early T cell activation using OVA-specific CD8+ OT-I and CD4+ OT-II T cells. After immunization, the team observed that antigens initially rapidly drained into the lymphatic sinus (LS). Although some smaller antigens traveled into the deep T cell zone via LN conduits soon after immunization, these antigens were rapidly cleared, presumably into the circulation. The majority of antigen appeared to enter the parenchymal LN space independently of LN conduits and via diffusion across the highly permeable LS floor. This diffusion-limited process generated an antigen concentration gradient with maximum antigen concentrations near the LS floor and lower concentrations in the T cell zone deep within the LN cortex. Conjugates of EαGFP to high molecular weight molecules were unable to enter the conduits, demonstrating the size dependence of this clearance mechanism.
To examine antigen uptake and presentation, Gerner et al. focused their attention on two specific subsets of LN-resident DCs: cDC1 cells, which mediate MHC I antigen cross-presentation to CD8+ T cells, and cDC2 cells, which specialize in MHC II antigen presentation to CD4+ T cells. The researchers confirmed their previous findings that LN-resident cDC1 and cDC2 cell populations occupy distinct regions of the lymph node, with the former localized within the deeper paracortical (T cell zone) region, and the latter mostly segregated in the regions near the LS. The antigen gradient determined the uptake by the asymmetrically-distributed DCs, with the centrally-located cDC1s acquiring significantly less antigen than the peripherally-situated cDC2s. The researchers confirmed that it was the location within the LN tissue, and not any cell-intrinsic uptake differences, that accounted for this phenomenon. Interestingly, they observed that the majority of antigen was associated with LN-resident DCs and not migratory DCs, suggesting either limited migratory DC involvement or rapid hand-off of antigen from migratory to LN-resident DCs.
To assess the immunological effects of these anatomical observations, the researchers used OVA immunization and in vivo imaging of CD8+ OT-I and CD4+ OT-II to demonstrate that the asymmetric distribution of cDC1 and cDC2 cells within the draining LN led to OVA-specific CD8+ T cell activation primarily deep within the LN paracortex, and CD4+ T cell priming occurring preferentially, but not exclusively, near the LS. Keeping in mind the antigen gradient across the LN, the researchers further demonstrated (using OVA titration) that effective MHC I presentation and CD8+ T cell activation required higher doses of administered protein than MHC II presentation and CD4+ T cell activation. This dose dependence also significantly affected the number of recruited CD8+ naive T cells, and ultimately, the CD8+ T cell clonal diversity as well as the magnitude of response.
Overall, the results of these imaging experiments demonstrate that lymphoid tissue microanatomy plays a significant role in the regulation of adaptive immune response, and suggest that antigen dosing and design should inform the development of vaccines.
This manuscript is accompanied by two informative videos displaying a 3D reconstruction of the dispersal of antigen within the LN and DC-mediated antigen uptake (Video 1) and of OT-I and OT-II cell clustering around DCs within the LN (Video 2).
by Anna Scherer




