Attempts to avoid systemic toxicity and Treg activation induced by IL-2-directed therapy has resulted in the development of various engineered immunotherapy products that specifically target IL-2Rβ/γ on CD8+ T cells and NK cells. However, these products have, so far, shown limited clinical efficacy. In a recent paper published in Cell Reports Medicine, Wu et al. report on preclinical testing of a new PD-1-targeted, receptor-masked IL-2 product with encouraging properties.
Wu et al. found that IL2v-Fc, which specifically targets IL-2Rβ/γ, results in systemic expansion of CD8+ T cells and NK cells, but does not specifically stimulate tumor-specific T cells in the tumor, limiting antitumor effects in vivo. Based on data suggesting that tumor-infiltrating CD8+ T cells expressing IL-2Rα and PD-1 may represent tumor-reactive T cells, an IL-2-based therapeutic was developed.
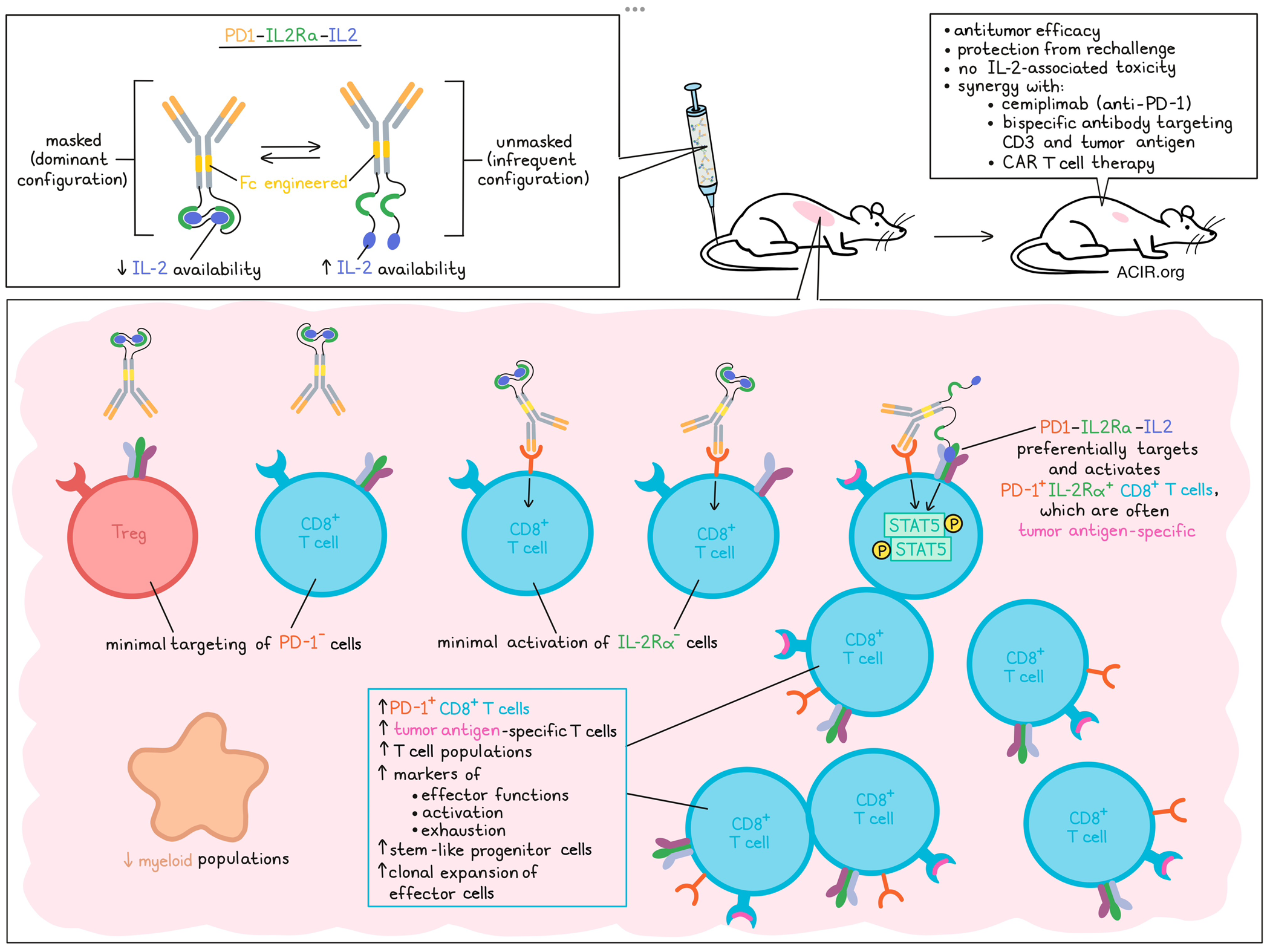
To target tumor-reactive cells, PD-1 was selected as the target for delivery of the masked IL-2 to selectively reconstitute IL-2 activity in PD-1+ T cells. To mask IL-2 during systemic circulation, a series of IL-2 binding domains fused to IL-2 were screened in an Fc fusion format, and the IL-2 receptor alpha (IL-2Rα) was selected based on its binding attenuation and biophysical properties. The resulting PD1-IL2Ra-IL2 construct was based on a hinge-stabilized IgG4 isotype that was modified to reduce or eliminate Fcγ receptor binding. PD1-IL2Ra-IL2 predominantly had a trans-sequestered confirmation, resulting in a “masked” configuration that reduced active IL-2 available to bind to IL-2 receptor subunits. The construct had stronger binding to IL-2Rα/β/γ+ cells than to IL-2Rβ/γ+ cells, and only infrequently “unmasked” to release active IL-2 to engage with these receptors.
The researchers assessed the role of PD-1 in the targeting of the masked IL-2 by comparing the IL-2 agonist activity of PD1-IL2Ra-IL2 with a non-targeting version (with anti-human MUC1 replacing anti-PD-1) in reporter cell lines. Both constructs had reduced potency as compared to recombinant IL-2 in triggering STAT5 activation in PD-1- cell lines. Increased PD-1 expression on the cells induced STAT5 activity in response to PD1-IL2Ra-IL2, but not the non-targeted version. Similar results were found in experiments with human peripheral blood mononuclear cells (PBMCs) in which PD-1+CD8+ T cells were selectively activated by PD1-IL2Ra-IL2.
Moving to in vivo experiments, Wu et al. first determined whether PD1-IL2Ra-IL2 could specifically expand PD-1+CD8+ T cells in the blood of human PD-1 knock-in mice. Indeed, therapy selectively expanded PD-1+CD8+ T cells, with minimal effects on PD-1-CD8+ T cells, while unmasked versions expanded all CD8+ T cells. Additionally, while unmasked versions induced body weight loss and pulmonary edema, daily administration of PD1-IL2Ra-IL2 did not result in IL-2-associated toxicity.
The antitumor activity of PD1-IL2Ra-IL2 was assessed in B16F10 melanoma, MCA205 fibrosarcoma, TRAMP-C2 prostate tumors, and Colon 26 and MC38 colorectal cancers. The PD-1-targeting antibody only binds human PD-1, so either the experiments were performed in human PD-1 knock-in mice, or a mouse surrogate mPD1-IL2Ra-IL2 molecule was used in C57Bl/6 or BALB/c mice. In all models, PD1-IL2Ra-IL2 had a strong antitumor effect. Depletion experiments showed that CD8+ T cells were necessary for tumor growth inhibition. Furthermore, therapeutic effects remained, even after blocking T cells egress from lymph nodes with the inhibitor FTY720, suggesting antitumor responses were induced by already-present tumor-infiltrating T cells.
T cell analysis of the spleen and tumor in MC38-OVA tumor-bearing mice after treatment showed that in the tumor, only PD-1+CD8+ T cells expanded, while Tregs did not. Conversely, in the spleen, both Tregs and PD-1+CD8+ T cells expanded. To assess whether memory was induced, mice whose MC38 tumors were eliminated by PD1-IL2Ra-IL2 treatment were rechallenged 97 days after the first tumor implantation, and none of the mice grew tumors, suggesting protective memory responses were induced by the therapy.
Further assessing the immune effects in tumors, the researchers performed single-cell RNAseq coupled to TCR V(D)J profiling in CD45+ cells from MC38 tumors after treatment. This revealed that treatment induced expansion of T cell populations and a reduction in myeloid populations. Most effects were detected on CD8+ T cells, and several PD-1+CD8+ T cell clusters were more abundant in the treatment group as compared to controls. These clusters also had increased expression of genes related to effector function and activation/exhaustion markers, suggestive of effector cells transitioning between various activation and differentiation states. Another cluster that was enriched after therapy consisted of CD8+ T cells expressing Pdcd1, Tcf7, and Bcl2, suggestive of stem-like progenitor cells.
Parallel single-cell TCRseq showed that therapy-induced effector CD8+ T cells had the most clonal expansion. In the TRAMP-C2 model, expanded tumoral T cells after treatment had an increase in the number and proportion of SPAS-1-specific T cells (a tumor antigen in this model).
Wu et al. then assessed combining other immunotherapies with PD1-IL2Ra-IL2 treatment. First, the combination of PD1-IL2Ra-IL2 with the PD-1 blocker cemiplimab was assessed in MC38-bearing human PD-1 knock-in mice. Treatment consisted of a single dose of PD1-IL2Ra-IL2 on day 9, followed by two injections of cemiplimab on days 14 and 19 (staggered dosing to prevent treatment interaction, as both drugs target PD-1. Anti-PD-1 alone was ineffective in this model, and while one dose of PD1-IL2Ra-IL2 resulted in some tumor control, most tumors relapsed, and only 2/8 mice had a complete regression. However, with the combination treatment, 8/10 mice achieved complete regression and long-term survival.
The second combination therapy tested was mPD1-IL2Ra-IL2 in combination with a bispecific antibody (BsAb) targeting CD3 and the antigen MUC16 in CD3/MUC16 double-humanized mice bearing syngeneic ID8-VEGF/huMUC16𝚫 tumors. Monotherapy with either of the reagents had limited effects on tumor control, but the combination improved control and resulted in 4/5 mice achieving complete tumor regression.
Finally, mPD1-IL2Ra-IL2 was tested in combination with CAR T cell therapy. Using the same model system as for the BsAb experiments, mice were lymphodepleted, implanted with tumors, and treated with anti-huMUC16 CAR T cells and mPD1-IL2Ra-IL2. This combination resulted in enhanced antitumor efficacy. When mPD1-IL2Ra-IL2 was combined with control CAR T cells, no therapy effects were observed, suggesting the activity of mPD1-IL2Ra-IL2 was dependent on the huMUC16 CAR T cells in these lymphodepleted mice. None of the three combination treatments resulted in toxicity.
Therefore, this new masked method of specifically targeting IL-2 to putative tumor-reactive PD-1+CD8+ T cells in the tumor shows potential to be effective in the clinic, while avoiding toxicity. In particular, PD1-IL2Ra-IL2 therapy was shown to be effective in combination with other immunotherapy strategies to further enhance antitumor responses, supporting broader clinical studies to treat challenging solid tumors.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, lead author Jiaxi “Chris” Wu and his team answered our questions.

What was the most surprising finding of this study for you?
When we started this project a few years back, the IL-2 cancer therapeutics development field was dominated by the IL-2Rβ/γ-biased strategy, aimed at reducing systemic IL-2 toxicity and preferentially activating CD8+ T cells and NK cells over Tregs. This "common wisdom" was challenged by our initial finding that a β/γ-biased IL-2 variant, despite inducing systemic expansion of CD8+ T cells and NK cells over Tregs as postulated, exhibited very limited antitumor efficacy due to its inability to specifically stimulate tumor-specific T cells. This unexpected finding prompted us to develop a new IL-2 therapeutic that maintains the ability to engage IL-2Rα, as described in this paper.
What is the outlook?
The next steps involve determining whether this research can translate into clinical benefits for patients with cancer. A first-in-human study has been initiated to test the safety and activity of PD1-IL2Ra-IL2 (REGN10597) in patients with advanced solid organ malignancies.
What was the coolest thing you’ve learned (about) recently outside of work?
Jiaxi “Chris” Wu: One exciting thing I learned recently outside my current work, though still related to science, is that my Ph.D. advisor, James Chen, was awarded the 2024 Lasker Basic Medical Research Award last week for discovering the DNA sensor cGAS. As a graduate student under James more than a decade ago, I was a major contributor to this discovery. I hope that my work throughout different stages of my career – past, present, and future – will ultimately benefit human health in some way.
Aaron Chang: Outside of work, I have been breakdancing as a hobby since I was a teenager. It has been great to see that breakdancing made the recent 2024 Olympic games. I learned that some dancers I practiced with over the years even represented team USA. I find more parallels between science and breakdancing than you might expect – you must be creative and think outside the box for both!
Nicolin Bloch: On a personal note, I recently discovered the humility of a friend when my husband found an Emmy award trophy in his living room and exclaimed, “Did you hide this all these years?” Indeed, he had. From a professional standpoint, I also recently realized that I had misunderstood the technology behind alphaLISA for years, despite being an avid user. It was a good reminder not to overlook details and to stay humble about what I know.
Ramandeep Bhavsar: I recently learned from my fitness journey that change doesn't happen overnight and isn't about perfection; it's about effort. I am dedicated to putting in the effort every day to improve, regardless of my motivation, whether at work, in the gym, or as a parent.
Supriya Patel: I've recently been learning a lot about home renovations as we buy and remodel our new home. It's fascinating to discover cutting-edge innovations that integrate advanced technologies, making everything smarter, more efficient, and hopefully more sustainable. Like a scientist, this process requires asking many questions, conducting thorough research, problem-solving, and being adaptable.




