T cell exhaustion is a major focus of research in cancer immunotherapy, and particularly in CAR T cell therapy, but still the exact mechanisms that drive T cells to exhaustion are not fully understood. Recently, Zhang, Zhang, and Qiao et al. developed an in vitro CAR hypofunction model and identified the AP-1 family transcription factor BATF – which different groups have reported to have conflicting functions – as a major driver of CAR T cell exhaustion. The results of this investigation were recently published in Cancer Cell.
To develop their hypofunction CAR T cell model, Zhang, Zhang, and Qiao et al. cultured T cells expressing the M28Z CAR (anti-mesothelin [MSLN] scFv, CD28 costimulatory domain, and CD3ζ intracellular domain) with NCI-H226 or OVCAR3 tumor cells, which express the target antigen MSLN. The M28Z CAR T cells induced robust tumor cell lysis upon co-culture, though at a low effector:target cell (E:T) ratio, CAR T cell mediated-lysis plateaued around day 4, despite the maintained expression of both the M28Z CAR on T cells and MSLN on tumor cells.
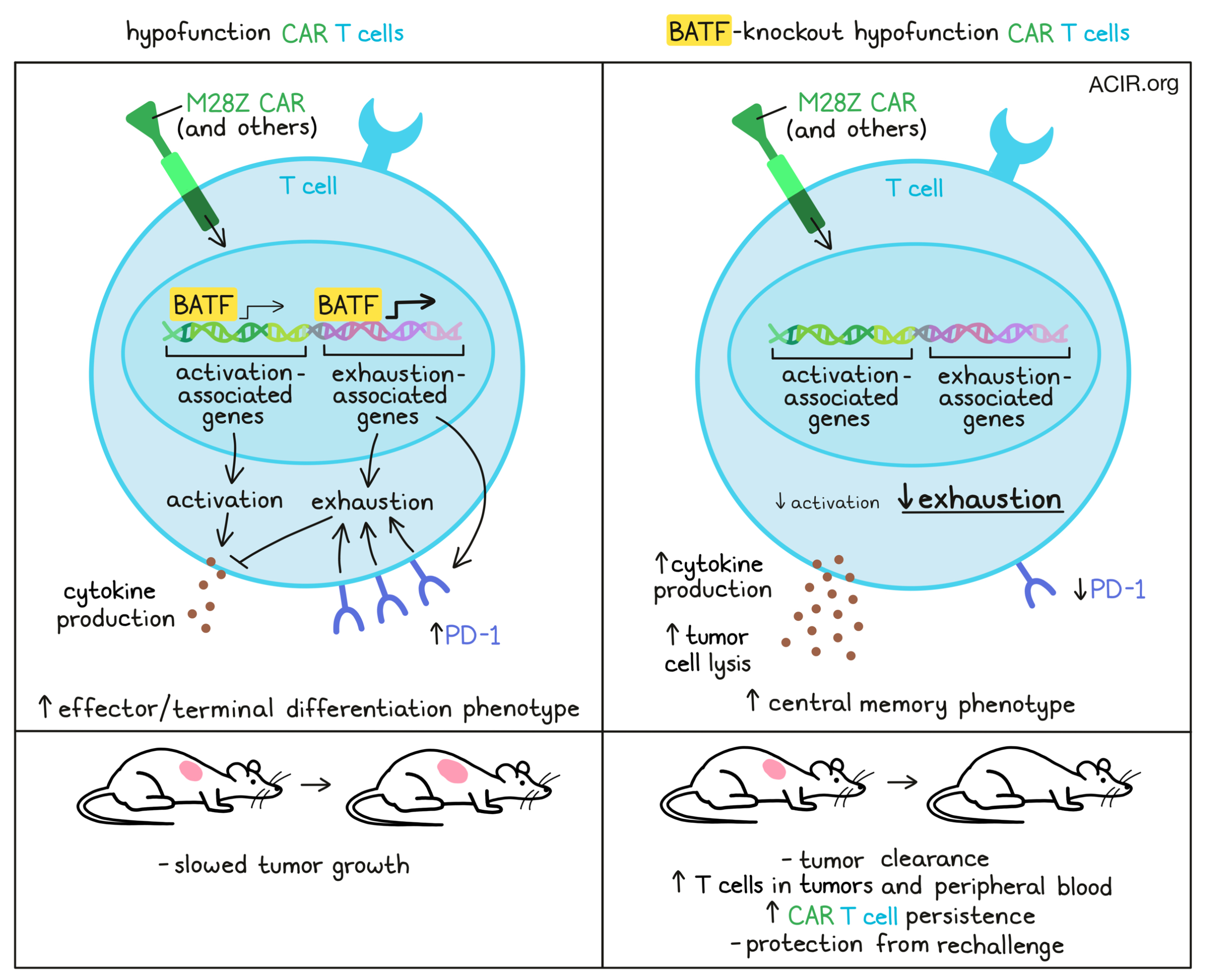
The researchers then compared “hypofunction” M28Z CAR T cells (from 7-day co-culture at a low E:T) to activated M28Z CAR T cells (from 1-day co-culture at a high E:T ratio), control “fresh” M28Z CAR T cells (cultured alone for 2 days), and control “unstimulated” M28Z CAR T cells (cultured alone for 7 days). Hypofunction M28Z produced fewer cytokines, showed increased expression of exhaustion-related genes, (e.g., PDCD1, HAVCR2, CTLA, and TIGIT), and showed reduced expression of activation-related genes (e.g., IFNG, IL2, TNFRSF9, and GZMB) compared to activated M28Z or controls. Gene set enrichment analysis (GSEA) further revealed that exhaustion-related genes identified in patients with cancer were enriched in the upregulated genes of hypofunction M28Z. Together, these results are indicative of a profound exhaustion phenotype in hypofunction M28Z.
Next, the researchers set out to identify genes that might contribute to hypofunction/exhaustion. After validating the function of PDCD1 by showing that its knockout improved CAR T cells’ ability to eliminate tumors, the researchers evaluated several less studied genes, including 19 genes with expression patterns that resembled PDCD1, and 11 genes that were selected for their upregulation in relation to T cell function. Of the genes screened, two stood out for their role in mediating exhaustion: BATF and IRF4.
To confirm the roles of BATF and IRF4 in CAR T cells, Zhang, Zhang, and Qiao et al. generated knockout models of each and showed that elimination of either gene led to increased cytokine release and tumor cell lysis, and to increased proliferation upon multiple rounds of tumor challenge. These effects were more pronounced in the BATF knockouts than in the IRF4 knockouts. Similarly, RNAseq and gene ontology analysis showed upregulation of genes enriched in T cell activation-associated pathways in both knockout models, but reduced expression of genes enriched in exhaustion-associated pathways only in the BATF knockout CAR T cells.
Testing whether this enhanced functionality would carry through to in vivo models, the researchers evaluated BATF-knockout CAR T cells and IRF4-knockout CAR T cells against control M28Z CAR T cells in cell line-derived xenograft (CDX) mice bearing NCI-H226 tumors. While standard M28Z and IRF4-knockout CAR T cells both slowed tumor growth compared to control-treated mice, this effect was significantly stronger in mice treated with BATF-knockout CAR T cells. Further, only the BATF-knockout CAR T cell treatment increased tumor-infiltrating T cells, which expressed IFNG and MKI67 by in situ RNA hybridization. Similar effects were observed in a patient-derived xenograft (PDX) model. In this setting, standard and IRF4-knockout CAR T cells only slowed tumor growth, while BATF-knockout CAR T cells cleared tumors in 4 of 5 mice. Further, higher levels of human CD3+ T cells were maintained in this group, and cured mice were able to clear tumors upon rechallenge, indicating durable protection. Similar results were also observed in a second PDX model,
To further validate the role of BATF in human CAR T cells, BATF was overexpressed by lentiviral transduction. This reduced the cytolytic capacity of CAR T cells upon multiple rounds of tumor challenge, while BATF knockout enhanced cytolysis. These effects were most pronounced when the E:T ratio was low, and with each additional cell challenge. Similar results were observed across in vitro and in vivo studies of 5 different CAR T cell types with different targets and costimulatory domains, as well as in murine OT-1 cells.
Next, the researchers performed RNAseq on CAR T cells after 3 rounds of in vitro tumor challenges, followed by ChIPseq and motif analysis of regions bound by BATF in CAR T cells. Comparing human exhaustion-related genes with those bound by BATF, the researchers found significant overlap. BATF was also found to directly bind to and upregulate PDCD1, increasing PD-1 expression and downstream exhaustion in BATF-overexpressing cells. In mouse models with overexpression of PD-L1 in tumors, BATF-knockout T cells showed enhanced antitumor activity. Interestingly, though BATF-overexpressing cells showed upregulation of genes related to proliferation, BATF-knockout cells were more abundant under exhaustion-inducing conditions, suggesting that the role BATF plays in inducing exhaustion is dominant over its role in inducing activation.
BATF has also been reported to play a role in the differentiation of effector T cells, and here the researchers found that BATF bound to and upregulated a number of effector genes (e.g., RUNX3, KLRG1, and TBX21), but also bound to and repressed genes involved in memory formation. In line with this, BATF-overexpressing cells showed more of an effector phenotype, especially in fresh or activated populations, while BATF-knockout cells were enriched for a central memory phenotype and memory T cell markers, especially after several rounds of tumor challenge. In vivo, TILs and peripheral blood from BATF-knockout CAR T cell-treated mice were both enriched for central memory cells, while those from mice treated with BATF-overexpressing cells were more terminally differentiated. By day 42, human CD3+ T cells could be detected in the peripheral blood of 5 out of 5 mice treated with BATF-knockout cells, but only 1 of the 5 mice treated with control CAR T cells, and none of the 5 mice treated with BATF-overexpressing CAR T cells, suggestive of enhanced persistence with BATF knockout.
Overall, these results help to clarify the role of BATF in orchestrating T cell activation, exhaustion, and differentiation. They also show that elimination of BATF can be used to enhance the functionality of CAR T cells for use against tumors, which could potentially translate to improved results in clinical applications in the future.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, lead author Haoyi Wang answered our questions.

What was the most surprising finding of this study for you?
The most surprising finding of this study is that genetically modifying BATF leads to different, sometimes opposite functional outcomes, depending on the specific experimental setup. BATF promotes T cell proliferation, however, it drives T cell exhaustion as well. Therefore, under a non-exhaustion condition (high CAR T cell to tumor cell ratio), overexpressing BATF leads to better CAR T cell proliferation and efficacy. However, under an exhaustion-inducing condition, overexpressing BATF deepens CAR T cell exhaustion, reducing cell proliferation and efficacy. This explains the discrepancy in previous studies, and highlights that the interpretation of a gene’s function could be highly context-dependent.
What is the outlook?
In this study, we established a primary human CAR T cell exhaustion model, which demonstrates a profound exhaustion phenotype. Using this model, we are currently performing larger-scale genetic screens and drug screens to identify genes and small molecules that could be used to rescue T cell exhaustion. On the other hand, we are combining BATF depletion with other modifications, such as knocking out TGFBR2 or ADORA2A to further improve the efficacy of CAR T cells against solid tumors.
What was the coolest thing you’ve learned (about) recently outside of work?
I have recently become a fan of audio books. Using a popular audio book reading app, any book can be directly played in my car audio system. Therefore, I have been reading many books during my everyday driving. This really gives me an opportunity to finish the books I always wanted to read, but had no time to before. The books I most recently enjoyed reading are “In search of memory”, “Journey to the Edge of Reason: the Life of Kurt Gödel”, “Shantaram”, and “Neapolitan Novels”.




