While the effects of immune checkpoint blockade on the tumor microenvironment (TME) are widely investigated, the combined effect of chemotherapy and checkpoint blockade is less clear. Further, although the Impassion130 trial in advanced triple-negative breast cancer (TNBC) combining nab-paclitaxel with anti-PD-L1 therapy led to clear patient benefits and product approval, a similar trial (Impassion131) combining paclitaxel with anti-PD-L1 therapy did not, indicating that important distinctions still need to be understood. Therefore, Zhang, Chen, Mo, and Hu et al. comprehensively investigated the effects of paclitaxel and anti-PD-L1 on immune populations in the TME in TNBC. Their results were recently published in Cancer Cell.
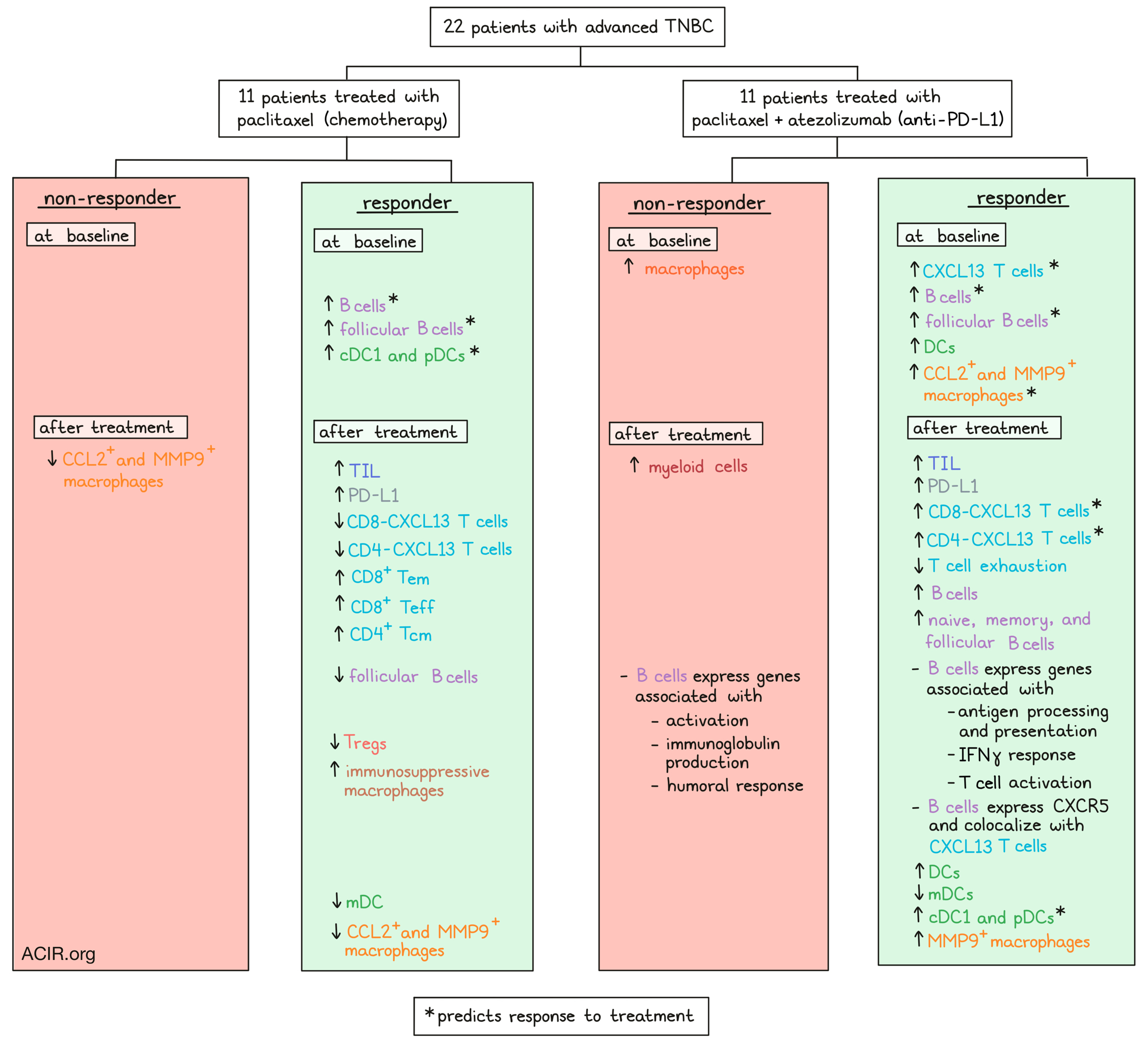
The study included 22 patients with advanced TNBC, of whom half received paclitaxel and the other half paclitaxel plus atezolizumab as first-line therapy. Multiple tumor and biopsy samples were collected. In tumor samples after treatment, responders had higher levels of TIL and PD-L1 expression. Exome sequencing of 78 tumor biopsies and blood samples obtained at baseline, four weeks after treatment initiation, and at the time of disease progression revealed the expected mutation burden for breast tumor samples. Single-cell transcriptome data showed that the main immune cell type that increased in responsive tumors after the combination treatment were B cells. In contrast, in nonresponsive tumors, the number of myeloid cells went up.
The researchers developed two indices, the predictive index (Pi), which measured the correlation between baseline cellular proportions with changes in tumor size, and the therapeutic index (Ti), which measured correlations between cellular proportion dynamics with tumor size changes. The Pi showed that B cells most accurately predicted favorable response to either treatment regimen, while the Ti showed that T cell changes correlated with therapy response to combination therapy.
The researchers then reclustered each major immune compartment. Two T cell clusters that stood out, CD8-CXCL13 and CD4-CXCL13, had a high expression of PDCD1, CXCL13, and exhaustion genes such as TIGIT, CTLA4, and LAG3. In addition to this exhausted phenotype, these subsets showed a high level of clonal expansion, suggesting these subsets may have tumor-reactivity potential.
After paclitaxel treatment, responders had lower levels of CD8-CXCL13, CD4-CXCL13, and Tregs, while CD8+ Tem, CD8+ Teff, and CD4+ Tcm rose. In contrast, when patients received the combination treatment, responders had higher levels of CD8-CXCL13 and CD4-CXCL13 cells, suggesting the CXCL13 populations were relevant for PD-L1 blockade. Using TCR sequences to trace the lineage origins of these populations, the researchers found that both pre-existing and newly infiltrated clones expanded, but the most prominent components were pre-existing clones.
The Pi analysis and IHC analyses also showed that the CXCL13 T cell populations were predictive for response to the combination treatment. In addition, TCGA data revealed that breast tumors had fewer infiltrating CD8+CXCL13+ T cells than melanoma or lung tumors, both of which generally respond better to checkpoint blockade.
After combination therapy, responders also had higher levels of CD19+ B cells. These B cells expressed CXCR5, the receptor for CXCL13, indicating a probable relationship with the CXCL13 T cell populations. In tumor samples of responders, CD19+ cells colocalized with CXCL13+ cells. The B cells enriched in responsive tumors highly expressed genes involved in the antigen processing and presentation, response to IFNγ, and T cell activation pathways. In contrast, the B cells in nonresponsive tumors were enriched for B cell activation, immunoglobulin production, and humoral response genes.
Naive, memory, and follicular B cell subsets were enriched in tumors responsive to combination therapy, but not in tumors responsive to paclitaxel alone. Responders to both therapy regimens had higher baseline levels of follicular B cells, and this population was associated with favorable clinical responses in the Pi analysis. The follicular B cells decreased in those responding to paclitaxel, while the population increased in those responding to combination therapy. The follicular B cells expressed high levels of CXCR5 and were highly correlated with the two CXCL13 T cell subsets, particularly after treatment, further indicating that these cells might interact.
Looking into the potential activators of the CXCL13 T cell populations, the researchers then assessed myeloid cells in the tumors. Responders exhibited higher levels of DCs at baseline and after combination treatment, but not before or after chemotherapy alone. Unsupervised clustering of the DCs revealed that higher levels of conventional DC1 (cDC1) and plasmacytoid DC (pDC) at baseline were associated with response to paclitaxel, but no baseline DC subsets were associated with response to combination therapy. Responders had decreased levels of mature DC (mDC) after paclitaxel, which correlated with cDC1. In those responding to the combination treatment, there was also a decrease in mDC, while cDC1 and pDC increased, and these populations were associated with improved clinical outcomes.
On the other hand, higher levels of macrophages were found at baseline in nonresponders to combination therapy. There was considerable heterogeneity in monocyte and macrophage populations, and the macrophages coexpressed canonical M1 and M2 signatures. In the TCGA BRCA dataset, high expression of CD8A and CXCL13 was correlated with CCL2 and MMP9-expressing macrophages and various DC subsets. In the single-cell RNA sequencing dataset, CCL2 and MMP9 macrophages were also highly correlated with the CXCL13 T cell subsets, and higher baseline levels were associated with response to combination therapy. The researchers also observed simultaneous enrichment of MMP9 macrophages and the CD8-CXCL13 subset in responsive tumors to combination therapy. In contrast, paclitaxel treatment alone decreased CCL2 and MMP9 macrophages, while immunosuppressive macrophages increased in responders, suggesting that paclitaxel may contribute to a stronger immunosuppressive phenotype.
The researchers then assessed changes in the transcriptome in CXCL13 T cells after therapy. In the CD8-CXCL13 subset, there was an upregulation in effector- and memory-related genes, transcription factors, costimulatory molecules, and HLA genes and integrins, while exhaustion-related genes were downregulated. This was reflected by a decrease in exhaustion score and an increase in effector memory score. Upregulated genes after treatment were involved in pathways regulating T cell-mediated cytotoxicity, antigen processing and presentation, and IFNγ-mediated signaling pathways.
Single-cell ATACseq analysis was performed to assess changes in chromatin accessibility. Chromatin accessibility dynamics of CD8-CXCL13 showed that effector properties enhanced after combination therapy, while the accessibility of exhaustion-related genes decreased, confirming the previous data.
This deep and longitudinal single immune cell data by Zhang, Chen, Mo, and Hu et al. suggest that paclitaxel chemotherapy and PD-L1 blockade have distinct effects on the tumor microenvironment, and that PD-L1 blockade may reverse some of the paclitaxel-induced immunosuppressive effects, increasing therapy efficacy and pointing the way toward more effective regimens
Write-up by Maartje Wouters, image by Lauren Hitchings





