Despite major successes with CD19-directed CAR T cell therapy in various hematological malignancies, not all patients respond. Given that CAR T cells (CART) upregulate immune checkpoint molecules, and that combination treatment with immune checkpoint blockade can enhance responses, Agarwal et al. assessed whether deletion of CTLA-4 and/or PD-1 from CAR T cells could improve therapy efficacy. Their results were recently published in Immunity.
The researchers manufactured CART19 cells from normal donor human T cells (WT) or from the donor cells in which CTLA4 and/or PDCD1 (encoding PD-1) was deleted using CRISPR/Cas9. In vitro, the CART19 with deletions in CTLA-4 and/or PD-1 had similar proliferation, memory phenotypes, levels of inhibitory receptors, and surface CAR expression as WT CART19. These CART also had similar levels of cytotoxic activity, degranulation, IL-2 production, and extracellular levels of TNFα, IFNγ, and GM-CSF as WT CART.
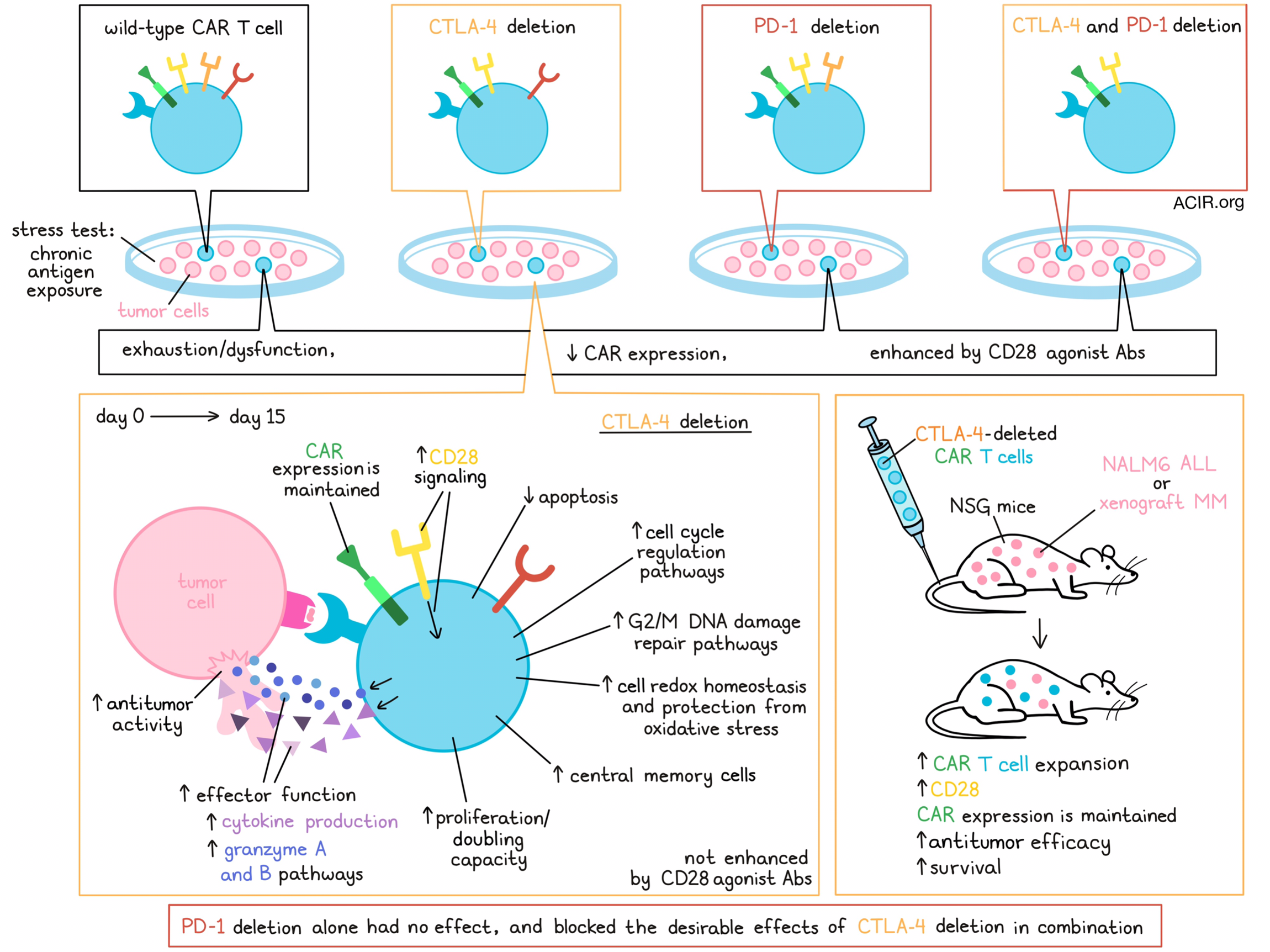
To determine whether these deletions could prevent CART dysfunction, the researchers made use of an in vitro stress test with chronic antigen exposure. The CART19 cells were repeatedly stimulated with CD19+ NALM6 cells at a high tumor-to-T cell ratio. WT CART19 became dysfunctional, whereas CTLA-4-deficient CART19 had increased doubling capacity and improved antitumor activity compared to WT CART19 or CART19 cells in which PD-1 or PD-1/CTLA-4 were deleted. The CTLA-4-deleted CART19 also had a higher proportion of central memory T (Tcm) cells, secreted more cytokines, and had fewer early apoptotic cells at the end of the stress test than the other CART19, suggesting better functionality.
Testing translatability to the clinical setting, when T cells from patients with chronic lymphocytic leukemia (CLL) were used for CART manufacturing, similar results were observed, with CTLA-4 deletion resulting in a higher percentage of Tcm cells, enhanced proliferation, persistence, cytokine production, and cytolytic activity than WT CART19 in the stress test. After the stress test, the researchers also noted Increased binding of antibodies targeting surface CD80 and CD86 to CTLA-4-deficient CART19 compared to WT cells, showing that these enhanced effects may be due to the prevention of CD28 signaling inhibition.
Since downregulation of surface CAR expression can also result in reduced functionality, the researchers explored CAR levels on CTLA-4-deleted CART19 in the dysfunction model. The levels of CAR were reduced in all variants after the stress test, except for the CTLA-4-deleted CART19, which maintained CAR expression.
Agarwal et al. moved to the NALM6 acute lymphoblastic leukemia (ALL) model in NSG mice to test the in vivo antitumor activity of the various CART19 cells. The CTLA-4-deleted CART19 induced improved tumor control, survival, and greater T cell expansion compared to WT and PD-1- or PD-1/CTLA-4-deficient CART19. Similar results were observed with CTLA-4-deficient versus WT CART19 derived from CLL patients. No signs of graft-versus-host disease were observed. The CTLA-4-deficient CART19 derived from patient samples expanded more in the periphery than WT CART19 on days 7 and 14. CD28 expression was higher on day 7, and CAR expression was maintained on days 7 and 14. These data were confirmed in a xenograft model of multiple myeloma (MM1.S), which was treated with edited BCMA-targeting CART. In this model, CTLA-4-deficient CART also had enhanced antitumor activity and led to improved survival, with more in vivo T cell expansion at day 7.
To determine which molecular pathways drive the enhanced efficacy of these CART, the researchers performed single-cell RNAseq of WT and CTLA-4-deleted CAR T cells on days 0 and 15 (endpoint) of the stress test. On day 0, three clusters were observed: terminal effector, proliferating, and effector memory T cells. On day 15, three additional clusters were detected: dysfunctional, terminally exhausted, and stressed and dying clusters. Compared to WT CART on day 15, CTLA-4-deleted CART had more proliferating and fewer stressed and dying cells. In WT CART on day 15, there was an upregulation of exhaustion genes and positive enrichment of several previously published exhaustion and dysfunction datasets, as well as a negative enrichment of intermediate and progenitor exhausted T cells. Similar analyses were performed on CART derived from CLL samples. Proliferation clusters were absent in WT cells, but were prominent in CTLA-4-deleted CART. Dysfunctional cells, on the other hand, were more abundant among WT CART on day 15, and even at baseline.
To determine what mechanisms are involved in the increased antitumor efficacy of CTLA-4-deficient CART, the researchers compared differentially expressed genes between WT and CTLA-4-deficient CART derived from healthy donors and CLL patients. CTLA-4-deficient cells upregulated Granzyme A and B signaling pathways, cell cycle regulation pathways, and G2/M DNA damage checkpoint regulation pathways after the stress test. Furthermore, genes involved in cellular redox homeostasis and protection of cells from oxidative stress were also upregulated. Additionally, expression of downstream targets of CD28, including GZMB, IL-2RA, and CDK6, were upregulated in CTLA-4-deleted CART.
To determine why CART deficient in both CTLA-4/PD-1 did not have enhanced antitumor efficacy, the researchers compared gene expression before and after the stress test between the various CART. There was much overlap between cells deficient in PD-1 versus. PD-1/CTLA-4, while WT versus CTLA-4-deficient cells had different profiles. Compared to the other groups, CTLA-4-deleted cells upregulated genes related to effector functions, proliferation, cytokine responses, central memory, and kinases, resulting in enrichment in pathways related to proliferation and cytotoxicity. In contrast, PDCD1/CTLA4-deleted cells, PDCD1-deleted cells, and WT cells were enriched for T cell exhaustion pathways.
The researchers hypothesized that the main impact in CTLA-4-deleted cells might be unopposed CD28 signaling, resulting in improved antitumor efficacy, while this phenotype is lost when PDCD1 is deleted. To test this, a CD28 agonist was added to the stress test experiment. This resulted in increased phosphorylation of ZAP70, LCK, and BTK/IK in all CART, except for the CTLA-4-deficient CART, which had high levels of phosphorylation of these genes without CD28 agonism that did not further improve upon adding the antibody. The addition of the agonistic CD28 antibody resulted in increased antitumor activity, proliferation, and CAR expression on day 15 in the WT, PD-1-deficient, and PD-1/CTLA-4-deficient CART, while it did not change the activity of the CTLA-4-deficient CART.
Together, these data show that with both healthy donor and patient T cells, CTLA4 deletion significantly improves antitumor activity, proliferation, and surface CAR expression of CART19, opening a novel path toward more effective CART therapy, potentially allowing for activity in patients who currently do not respond to this therapy and extending durability in current responders.
Write-up by Maartje Wouters, image by Lauren Hitchings




