Tumors with a low mutational burden generally respond poorly to immunotherapy. Given that radiotherapy can induce mutations in tumor cells and has been found to synergize with immunotherapy, Lussier, Alspach, and Ward et al. explored whether radiation of low mutation burden tumor cells induces neoantigens that can be recognized by T cells to improve checkpoint therapy responses. Such findings might contribute to understanding the synergy between non-curative radiotherapy and immunotherapy, and were recently published in PNAS.
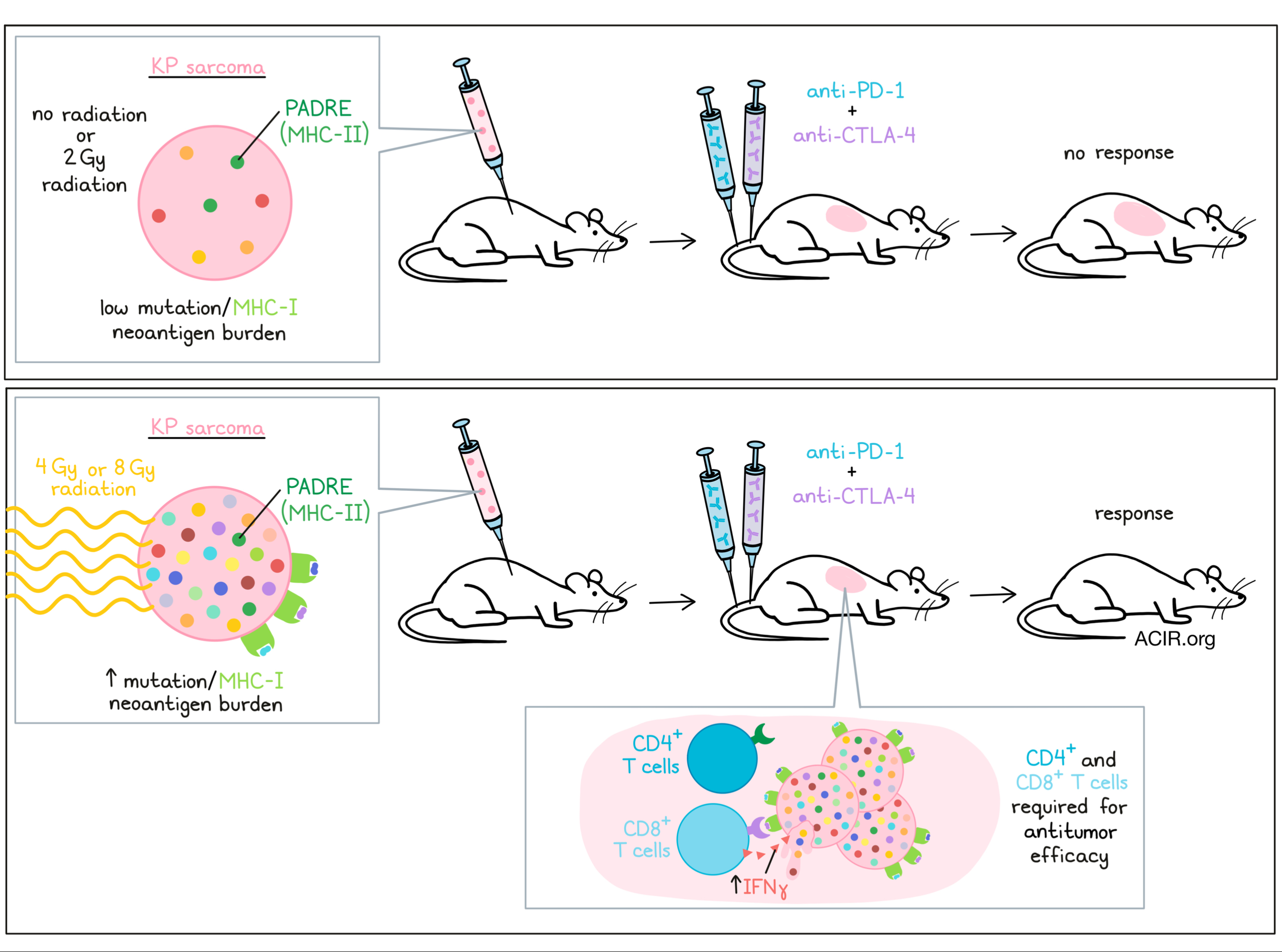
Previously, the researchers had shown that a cell line from the genetically engineered KrasLSL-G12Dxp53fl/fl mouse model formed sarcomas (KP sarcomas) that were insensitive to checkpoint blockade therapy due to a low mutational burden. Building from their previous work, the researchers selected two independent KP sarcoma cell lines (KP9093 and KP9032) to assess mutational burden and immunogenicity. They found only 14 and 12 somatic mutations on these cell lines, and none of the mutations were predicted to form MHC-I neoantigens. Both cell lines produced tumors insensitive to combination anti-CTLA-4/anti-PD-1 treatment, and no immunologic memory was formed, as mice whose tumors had been resected developed new tumors upon rechallenge. When the antigen ovalbumin was ectopically expressed in the cells, the tumors were rejected, suggesting that the lines have no or too few tumor antigens to elicit immune responses.
Lussier, Alspach, and Ward et al. engineered the KP9093 cells to express the pan MHC-II-restricted epitope PADRE to increase sensitivity to the presence of MHC-I antigens, based on their prior work(1). Even with a strong MHC-II antigen, the engineered cells also grew progressively in WT mice, did not respond to checkpoint blockade, and did not induce memory responses. To assess the effects of radiation, the cells were irradiated in vitro with either 2, 4, or 9 Gy, after which they were cultured for two weeks and injected into mice. Again, tumors grew progressively in both immunodeficient Rag2-/- mice and WT mice treated with control antibodies. However, the lines that received 4 or 9 Gy radiation treatment resulted in tumors sensitive to anti-CTLA-4/anti-PD-1 treatment, with 82% and 60% of mice rejecting tumors, respectively. The lower dose of radiation did not induce these effects.
To assess differences in responses, lines treated with 4 or 9 Gy were cloned, and single-cell clones were expanded into cell lines. These cloned lines were inoculated in the WT and Rag2-/- mice and the mice were treated with checkpoint blockade. Again, all lines grew progressively in the Rag2-/- mice, but of the WT mice receiving lines treated with 4 Gy, 26% rejected the tumor without checkpoint blockade, while 78% of mice rejected tumors with treatment. For the 8 Gy-radiated lines, the rejection rates were correspondingly 33% and 83%, suggesting this radiation regimen induced susceptibility to checkpoint blockade.
To evaluate the characteristics of these now immunotherapy-sensitive tumors, one representative line (KP.PAD.4G1) from the 4Gy cells that grew progressively in immunodeficient mice and control WT mice, but was rejected when WT mice were treated with checkpoint blockade, was selected for in-depth assessment. Depleting either CD4+ or CD8+ T cells in KP.PAD.4G1 tumor-bearing mice resulted in a loss of sensitivity to dual checkpoint blockade, suggesting both T cell subsets were required for the effects. Additionally, these cells were sensitive to single treatment of anti-CTLA-4 but not anti-PD-1, suggesting enhanced induction of T cell responses was responsible for the increase in therapy response. The mice rejected a rechallenge with the same cell line, but not the parental line or other clonal lines treated with 4 Gy radiation, suggesting each clone developed a specific set of mutation-induced antigens.
Subjecting the KP.PAD.4G1 cells to whole-exome and RNA sequencing and variant calling revealed the cells had 19 irradiation-induced missense mutations. Using MHC-I tetramer staining based on the epitopes predicted from these mutations, the researchers found CD8+ T cells in TILs from untreated tumors that were specific for nine of the mutations. To assess their reactogenicity, CD8+ T cells were stimulated with splenocytes that were pulsed with each tetramer-positive peptide. ELISPOT assays showed that four of the peptides elicited T cell IFNγ production. TILs from a mouse treated with CTLA-4 blockade had an even higher IFNγ response for one of these antigens.
Asking whether the radiation-induced effects depended on these antigens and not on other radiation-induced changes in the tumor cells, the authors enforced the expression of the four neoantigens individually in the parental (non-irradiated) cell line and repeated the checkpoint blockade experiments. Expression of two of the antigens resulted in sensitivity to checkpoint blockade, and expression of two other antigens prolonged survival in mice.
Finally, the researchers assessed whether these radiation-induced neoantigens were protective as a vaccine. The tumor lines with each of the four individual neoantigens were lethally irradiated and injected as a vaccine into WT mice. Ten days later, the mice were then challenged with the KP.PAD.4G1 line (which endogenously expressed each of the 4 neoantigens), resulting in tumor rejection in 80% and 60% of mice for two antigens, and delayed tumor growth for the two other antigens. When mice were vaccinated with either the unirradiated parental line (containing the MHC-II epitope), KP.PAD-4G1, or an antigenically irrelevant clonal line before being challenged with the bulk parental line that was irradiated with 4 Gy, only those mice vaccinated with the KP.PAD.4G1 clone derived from the parental line completely rejected the tumor. These findings suggest that neoantigens in the clonal cell line can promote rejection of the corresponding irradiated tumor cell line.
Overall, the data presented provide a mechanism of action based on the generation of immunogenic neoantigens to explain the synergistic effects of radiotherapy and checkpoint blockade, even when such neoantigens may be subclonal, and suggest that more comprehensive evaluation of parameters, such as dose, relative timing, and duration, may optimize the utility of this combination approach.
Write-up by Maartje Wouters, image by Lauren Hitchings
1MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019 Oct 23.
Alspach E., Lussier D.M., Miceli A.P., Kizhvatov I., DuPage M., Luoma A.M., Meng W., Lichti C.F., Esaulova E., Vomund A.N., Runci D., Ward J.P., Gubin M.M., Medrano R.F.V., Arthur C.D., White J.M., Sheehan K.C.F., Chen A., Wucherpfennig K.W., Jacks T., Unanue E.R., Artyomov M.N., Schreiber R.D.
Meet the researcher
This week, first co-author Danielle Lussier-Talbot answered our questions.

What prompted you to tackle this research question?
As we consider frontline immunotherapies in combination with standard-of-care treatments, we need to understand how standard-of-care treatments are changing the tumor microenvironment. We observed that glioblastoma patients treated with temozolomide had recurrent tumors that were often hypermutated, and we knew that highly mutated tumors tend to respond to checkpoint blockade treatments at a higher rate. We wanted to ask the question “if mutagenic standard-of-care treatments are inducing therapeutically relevant neoantigens, can they be targeted by the endogenous immune response and lead to effective tumor clearance?”. If so, it was important to consider the combination treatment regimen between standard-of-care treatments and immunotherapies, specifically if standard-of-care therapies are inducing neoantigens driving some of the synergy.
What was the most surprising finding of this study for you?
Standard-of-care treatments induce subclonal neoantigens among the tumor cells. We were surprised to see that vaccination with radiation induced subclonal neoantigens protected against a heterogeneous tumor, in which only a subset of cells express radiation neoantigens. Therefore, while subclonal neoantigens are weaker than clonal neoantigens in driving effective antitumor immune responses, these radiation-induced subclonal neoantigens were still capable of driving tumor rejection in a vaccination setting.
What was the coolest thing you’ve learned (about) recently outside of work?
There are more twins today than ever before.





