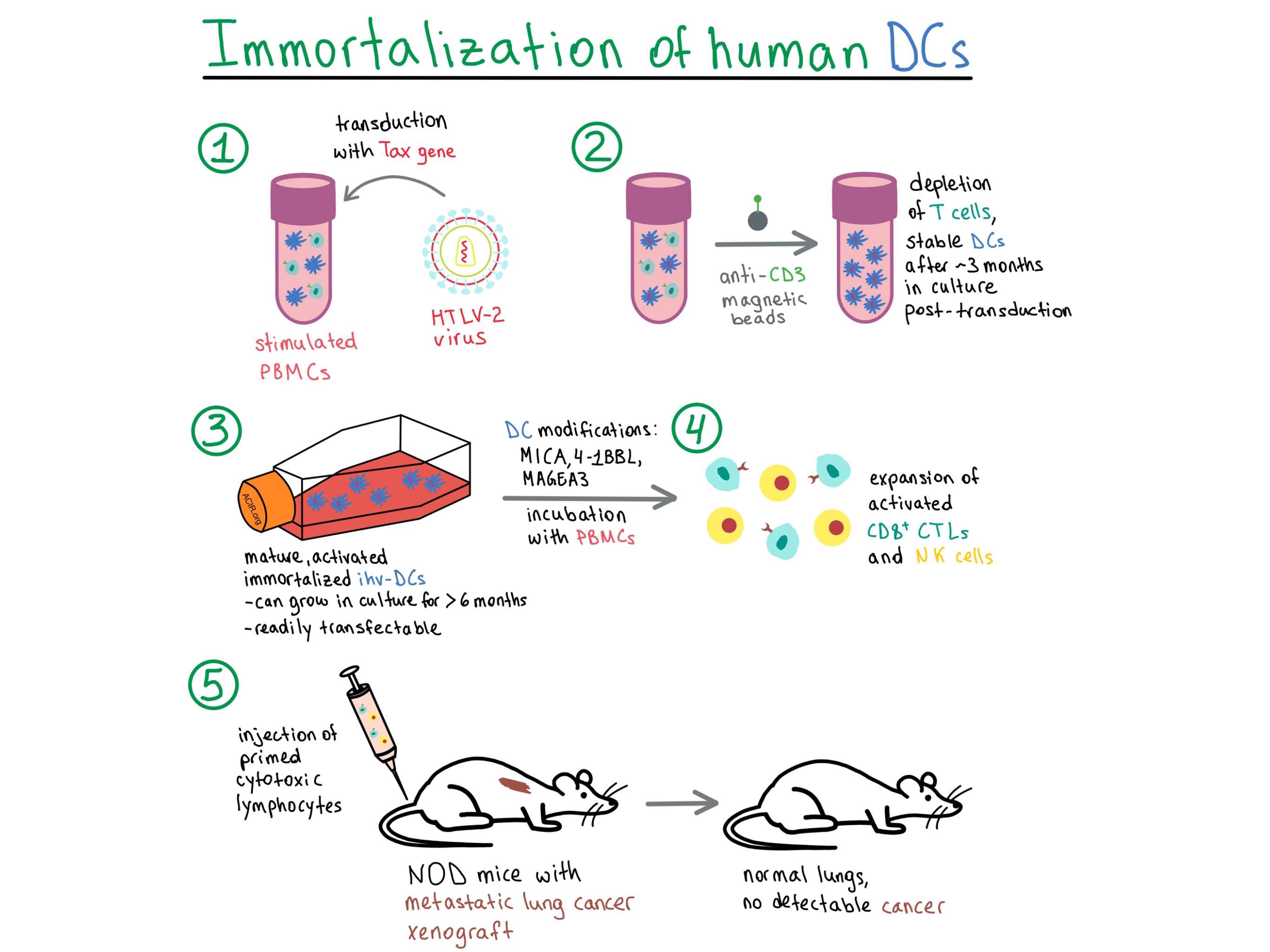
Current methods for preparing dendritic cells (DCs) for cancer vaccine immunotherapy, utilizing antigen-loaded monocyte-derived DCs (MoDCs) that are activated via a complex process, result in DCs that are difficult to maintain in culture, have low growth potential, and require repeated preparations for several rounds of vaccine delivery. In a paper recently published in PNAS, Wu et al. developed a method for establishing immortalized and constitutively activated human primary blood dendritic cell lines (ihv-DCs) that utilizes the viral protein Tax, a transcriptional regulator from human T cell leukemia virus type 2 (HTLV-2), which is not known to be pathogenic in humans.
To begin, Wu et al. transduced stimulated peripheral blood mononuclear cells (PBMCs) with Tax and, two to three weeks later, negatively selected for the CD3 marker to deplete T cells. Two cell lines, named ihv-DC1 and ihv-DC2, grew continuously in culture for over 6 months, and displayed DC-related markers (CD11c, CD141, CD205) and DC maturation and activation markers (CD83, CD80, CD86, CD70, CCR7, HLA-DR), with an immortalization rate of about 20%. To separately validate the ability of Tax to promote DC growth, the researchers in parallel generated MoDCs by stimulating adherent monocytes from PBMCs with GM-CSF/IL-4. The MoDCs that presented dendrites were transduced with Tax, detached during culture, and maintained proliferation for up to 3 months, but were not immortalized. Since the MoDC-Tax cells had a mature and activated phenotype, this experiment validated the ability of Tax to promote the growth of DCs in standard culture conditions.
Although various TLR receptors were upregulated in Tax-transduced DCs, they were no longer required for inducing maturation and activation in these constitutively activated DC lines that already persistently expressed high levels of costimulatory molecules. Both ihv-DC lines and the Tax-transduced MoDCs produced the inflammatory cytokines IL-1A and TNFα, as well as IL-15, which is involved in the development of cytotoxic CD8+ T lymphocytes (CTLs). Thus, the cytokine and TLR expression in transduced DC lines was similar to that in DCs generated by conventional methods. In addition, the transduced DCs upregulated the pro-survival protein Bcl-xL, as well as other growth-promoting, signaling, and cell cycle progression pathways, pointing to their immortalization.
Aiming to enhance antigen presentation of a desirable target epitope, the team transduced ihv-DC2 cells with a fusion construct encoding pTERT, which consisted of a fragment from human telomerase reverse transcriptase (hTERT) linked to a proteasomal target sequence of IκBα. hTERT contained six HLA-A2-restricted CTL epitopes and was degraded within DCs to facilitate epitope presentation. Mixing ihv-DC2-pTERT cells with autologous PBMCs led to priming of autologous CD8+ T cells, which produced large amounts of IFNγ and TNFα. However, the lymphocyte expansion was modest. To enhance the immune response to hTERT, the researchers introduced two HLA-DRB1 molecules into the ihv-DC2-pTERT cells. Such modified alloreactive DCs activated CD4+ T cells, which in turn enhanced the proliferation of CD8+ CTLs, enabling lysis of TERT+ bone osteosarcoma, melanoma, and lung cancer cells in an HLA-A2-restricted manner.
Because cancer cells are often heterogeneous in their expression of HLA molecules, it is desirable to induce both an HLA-specific CTL response and an HLA-independent natural killer (NK) cell response. To this end, Wu et al. engineered ihv-DC1 cells to express high levels of the NK cell activators MICA and 4-1BBL, in addition to the endogenously expressed CD58, in order to efficiently activate NK cells. These modified DCs were also transduced with the HLA-A2.1 molecule and made to overexpress the testis antigen MAGEA3, which is present in many cancer types. The altered DCs (ihv-DC1.2-MAGEA3) were incubated with HLA-A2+ matched naive PBMCs from various donors for at least 2 weeks, leading to stimulation of both CTLs and NK cells. The primed CTLs were able to lyse various types of MAGEA3+ cancer cells in an HLA-A2-restricted manner, while the activated NK cells were highly cytotoxic to HLA-A2- cancer cells.
Moving on to in vivo experiments, the researchers infused NSG mice with A549-Luc/A2.1 cells to establish lung metastases, and then treated the mice with ihv-DC1.2-MAGEA3-primed cytotoxic lymphocytes. The mice in the treatment group had lungs that were normal in appearance and weight compared with the enlarged lungs found in the control group. Cancer cells were undetectable in the lungs of treated mice. Both CTLs and NK cells were found in the lung tissues of treated mice, and the CTLs were also detected in the spleens and lymph nodes, and weakly in the livers.
In summary, Wu et al. developed a method to establish immortalized and constitutively activated human primary blood DC lines, and explored various modifications to the DCs to enhance their immunotherapeutic potential.
by Anna Scherer




