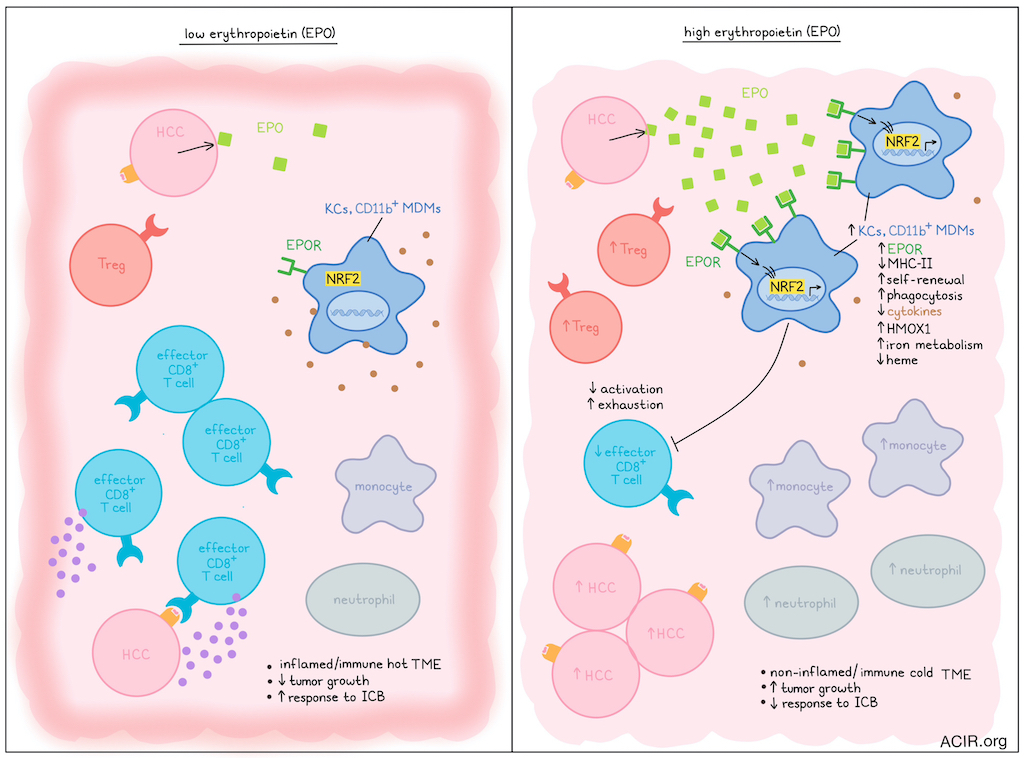
While some biomarkers, such as low tumor mutational burden, have been associated with immune-cold tumors that respond poorly to immune checkpoint blockade (ICB), the mechanisms underlying the immunosuppressive tumor microenvironment (TME) remain largely unknown. Assessing this question, Chiu et al. recently published their findings on the influence of erythropoietin (EPO) in defining this immunotype in Science.
The researchers focused their work on hepatocellular carcinoma (HCC) and developed several murine models with tumors with an inflamed or non-inflamed TME using genome editing approaches. Keap1KO tumors were inflamed tumors, with high numbers of infiltrating CD8+ effector memory T cells (Tem), and were responsive to ICB. Non-inflamed models included Trp53KO and PtenKO tumors, which had low T cell infiltration and did not respond to ICB. Surprisingly, the non-inflamed models exhibited splenomegaly and elevated levels of EPO.
Evaluation of HCC samples from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium suggested that increased EPO mRNA was associated with poor survival, macrovascular invasion, and poorer tumor differentiation. Further, CIBERSORTx analysis showed that EPO overexpression correlated with higher levels of Tregs and immunoregulatory M0-resting macrophages in the TME.
To assess the role of EPO in the TME, the EPO gene was knocked out in Trp53KO HCC tumors. EPO-KO HCC tumors demonstrated decreased tumor burden, with increased infiltration of effector CD8+ T cells and decreased infiltration of neutrophils and monocytes. A model with orthotopically implanted Hepa1-6 HCC cells had highly inflamed tumors that regressed spontaneously due to T cell responses. When the tumor cells were edited to overexpress EPO, tumors continued to grow, and the TME shifted from T cell-rich to myeloid cell-rich, with a lower proportion of activated tissue-resident memory T cells and a higher proportion of Tregs. When Tregs were depleted in this model, tumors regressed, suggesting Tregs were involved in the EPO-mediated immune effects.
The researchers then assessed EPO receptor (EPOR)-expressing immune cells in HCC. In the Trp53KO HCC model, EPOR+ cells constituted three subsets of macrophages: CD11bhi monocyte-derived macrophages (MDMs), CD11blo MDMs, and Tim4+ macrophages, which were defined as Kupffer cells (KCs). Of these three subsets, CD11blo MDMs and KCs expressed the highest levels of EPOR and had lower MHC-II. In the Hepa1-6 EPO-producing model, the overall frequency of EPOR+ macrophages in the liver was not increased, but the Tim4+ KC population expanded. While normal livers contained limited KCs, EPO expression in tumors resulted in a large increase in the percentage of KCs, suggesting these KCs were differentiated in the liver from monocytes or MDMs. EPOR expression on macrophages was higher in tumor than in adjacent non-tumorous tissue. Isolated EPOR+ macrophages from fresh human liver tumor tissue were subjected to RNAseq, which revealed enrichment in genes related to self-renewal and phagocytosis functions of KCs.
To test the hypothesis that EPOR+ macrophages mediate the effects of EPO in these tumors, the researchers developed LysM-driven EPOR deletion in mature myeloid cells (Epor𝚫LysM mice). In this model, mice with non-inflamed tumors had improved outcomes, while inflamed (low EPO) tumors were unaffected. The non-inflamed tumors showed linear growth in WT mice, while the Epor𝚫LysM and Keap1KO models had oscillating growth kinetics, suggesting active immune surveillance, which became linear growth in the Keap1KO model when recombinant EPO was administered. Further, neither EPO-secreting tumors in the Epor𝚫LysM model nor EpoKO tumors in WT mice could escape immunosurveillance, suggesting both EPO and EPOR-expressing macrophages are involved in the immunosuppression. Additionally, EPO-overexpressing Hepa1-6 tumors in the Epor𝚫LysM model regressed. Finally, administration of liposomes loaded with EPOR-targeting siRNA led to tumor regression of established non-inflammatory tumors, suggesting EPOR signaling was required to maintain the immunosuppressive TME.
Chiu et al. then assessed EPO-induced changes to the TME of Trp53KO tumors in the Epor𝚫LysM model. These tumors had higher proportions of effector T cells and macrophages with high MHC-II expression. CD8+ T cell depletion abrogated the spontaneous tumor regression and decreased survival. Epor𝚫LysM mice had high levels of early activated PD-1+TNFα+CD8+ Tem subsets in the TME, while in the WT mice, mainly terminally exhausted or bystander CD8+ T cells were found. In the Trp53KO model, EPOR ablation in macrophages and anti-PD-1 ICB resulted in synergistic antitumor effects, with all mice clearing the tumor. This effect could also be achieved by pharmacological inhibition of EPO/EPOR signaling using recombinant mouse EPOR-Fc chimera protein.
When Tim4+ macrophages from mice with Hepa1-6 with empty vector (KCEV) or EPO-overexpressing (KCEpo) tumors were assessed by bulk RNAseq, a distinct transcriptomic profile was found, with downregulation of inflammatory responses and cytokine secretion in the KCEpo tumors. Among the most upregulated genes in KCEpo were genes involved in scavenger receptor-mediated ligand binding and uptake, histone trimethylation, resolving acute inflammation, and cellular iron metabolism, while downregulated genes were related to antigen presentation, inflammation, response to external stimuli, protein folding, and cell activation. To determine whether EPO similarly regulates human HCC macrophages, TCGA data were assessed based on the Top 50 upregulated genes in KCEpo. In human tumors, EPO expression correlated with this KCEpo signature. KCEV contained gene signatures similar to human CD11c+ antigen-presenting KCs, while KCEpo resembled human ILIRB5+ metabolic and immunoregulatory KCs.
Genes enriched in KCEpo were found to be regulated by transcription factors related to hematopoiesis and stem cell biology. Many of the enriched genes are involved in iron metabolism and antioxidation, and are under the control of NRF2, and EPO stimulation induced NRF2 nuclear localization in KCs of HCC-bearing mice. Heme oxygenase 1 (HMOX1), which is involved in the breakdown of heme, was the most significantly upregulated gene in EPO-stimulated KCs. Heme is essential for multiple pro-inflammatory properties of macrophages. EPO stimulation reduced heme levels in KCs in the Hepa1-6 model, and EPOR deletion increased intracellular heme levels in tumoral macrophages in the Trp53KO and PtenKO HCC models (non-inflamed). In Nrf2r𝚫LysM mice, which lack NRF2 expression in macrophages, non-inflamed tumors progressed more slowly and exhibited greater CD8+ Tem infiltration, suggesting that EPO/EPOR signaling impacts macrophage phenotype through NRF2 activation and heme metabolism.
Together, the data suggest an important role of EPO/EPOR signaling in establishing immune-cold TMEs. If this mechanism can be targeted in patients, it may work synergistically with ICB to improve outcomes in immune-cold hepatic tumors, as well as for other largely immunotherapy-resistant tumors.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author David Kung-Chun Chiu and lead author Edgar Engleman answered our questions.
What was the most surprising finding of this study for you?
It is quite a surprise that erythropoietin (EPO), a hormone that has not only been studied for many years, but used clinically since 1989 for the treatment of chronic anemia, has a second distinct and fundamental function that may be more important than the first one — i.e., regulation of the immune response. (Unexpected results, obstacles, big challenges, etc...)
What is the outlook?
We believe that targeting EPO and/or its receptor (EPOR) on antigen-presenting macrophages and dendritic cells is likely to become an effective therapeutic strategy for the treatment of a wide range of cancers.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
EE: My advice would be to always try to tackle major questions, the answers to which are going to have far-reaching consequences for the field and for patients with cancer.




