Circadian rhythms allow organisms to operate on 24-hour cycles and coordinate periods of activity and rest in response to light and dark. These oscillations, controlled by both internal and external “clocks”, have also been shown to impact various aspects of the immune system, including responses to vaccines and tumor immune infiltration. To extend these observations, Wang and Zeng et al. recently evaluated the impacts of time of day on tumor microenvironments, T cell therapies, and immune checkpoint blockade to determine whether altering something as simple as the treatment time could play a role in immunotherapy outcomes. Their results were recently published in Cell.
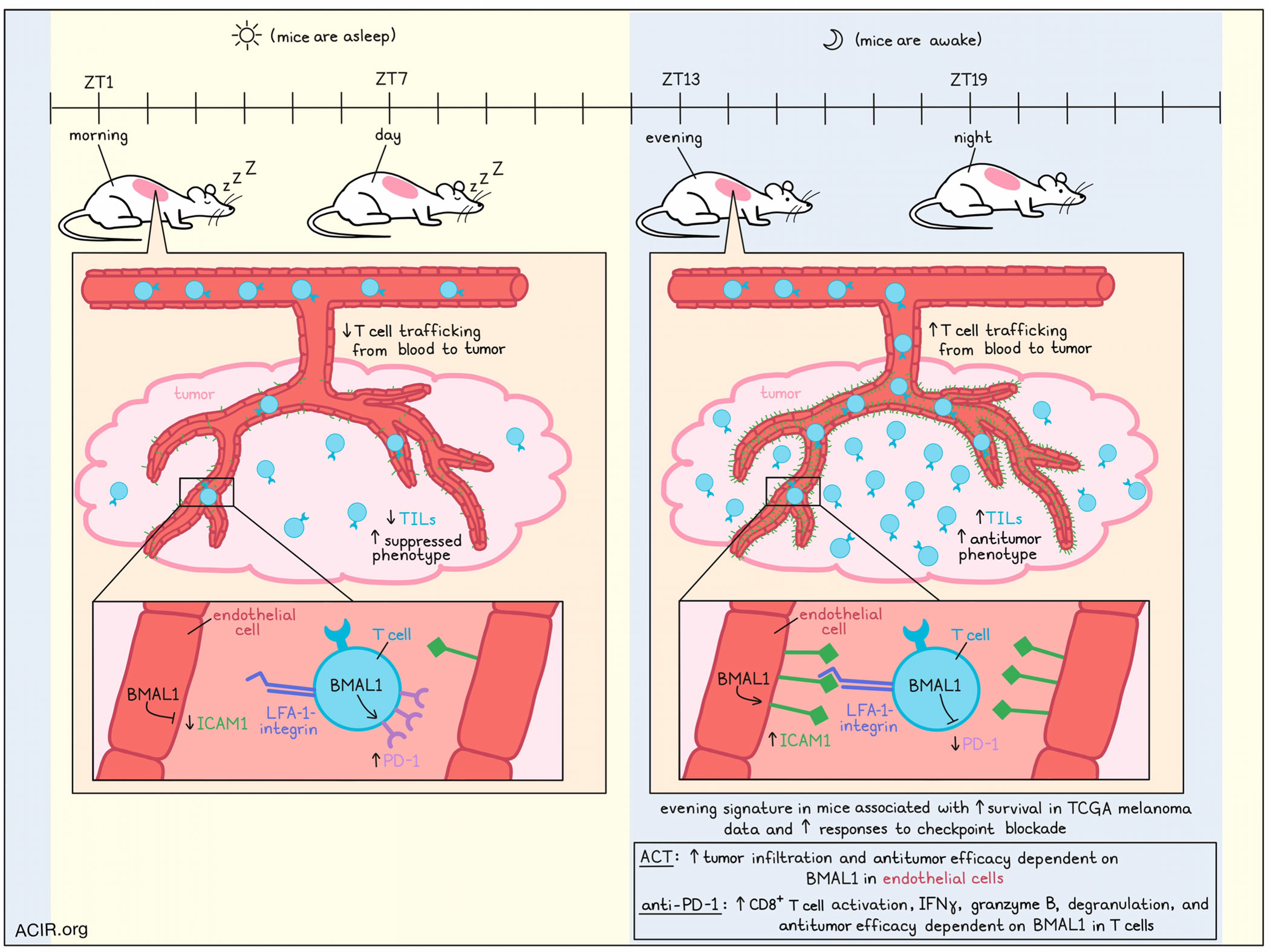
To begin, Wang and Zeng et al. used mice bearing B16F10-OVA tumors to evaluate changes in the tumor microenvironment at different times of day, which were defined based on 12 hours of light and 12 hours of dark. Tumors were implanted at the same time of day, and were harvested 12 days later at different times of day, defined as Zeitgeber time 1 (ZT1; morning; the first hour of light after a dark cycle), ZT7 (day), ZT13 (evening), and ZT19 (night), though it is important to note that mice are nocturnal, with their active period beginning at in the evening and their rest period beginning in the morning. In tumor samples, the number of TILs was highly dependent on the time of day, peaking in the evening. Time-of-day differences were apparent in CD4+, CD8+, NK1.1+, CD11b+Ly6C+ cells, CD11c+MHCII+, and CD19+ cells, but not CD11b+F4/80+ cells or CD11b+Ly6G+ cells. Similar results were observed in a spontaneous melanoma model. This pattern was maintained in mice under conditions of total darkness, suggesting some intrinsic circadian regulation, but were inverted in mice adapted to swapped light/dark schedules, and were lost when mice were subjected to an acute jetlag protocol, suggesting that changes in external light and dark cues can rewire circadian rhythms.
Investigating the mechanisms underlying circadian changes in TILs, the researchers found that proliferation, apoptosis, and egress were similar across time points, but that LFA-1-integrin-dependent leukocyte trafficking from the blood to the tumor varied by time of day, with more cells reaching the tumor in the evening. Looking at the TME itself, the researchers noted pro-migratory properties in the evening, with endothelial cells expressing higher levels of the intercellular adhesion molecule ICAM1 compared to in the mornings. Conditional knockout of the circadian clock gene Bmal1 in endothelial cells abrogated time-of-day differences in ICAM1 expression, along with differences in TIL numbers, suggesting that this circadian rhythm was intrinsic to endothelial cells.
Looking at how time-of-day differences in the TME might impact responses to adoptive cell therapy, which relies on effective tumor infiltration, Wang and Zeng et al. evaluated treatment of B16F10-OVA tumors with transferred OT-I cells. Notably, more transferred cells made their way into tumors, and tumors were better controlled when cells were administered in the evenings rather than in the mornings – an effect that was abrogated with deletion of BMAL1 in endothelial cells. Similar results were observed when CD19-targeted human CAR T cells were adoptively transferred into NSG mice engrafted with a human DLBCL cell line. Here, treatment in the morning showed almost no effect on tumor control, while treatment in the evening reduced tumor volume and was linked to increased CAR T cell homing.
Assessing whether the phenotypes of TILs were also time-of-day-dependent, the researchers performed scRNAseq on immune infiltrates of day 12 B16F10-OVA tumors, and found that circadian clock genes were expressed rhythmically in all leukocyte subsets, with Per1 exhibiting the most dramatic oscillation, peaking in the evening. Each immune cluster exhibited a distinct oscillatory gene signature. Most rhythmically expressed genes were associated with metabolism, but differences in cell adhesion, differentiation, and activation were also notable.
Further analysis of CD8+ T cells specifically showed that ratios of CD8+ T cell clusters that were considered anti- or pro-tumor peaked in the evening and troughed in the mornings, as did ratios of non-exhausted to exhausted CD8+ T cells, and ratios of effector memory to exhausted CD8+ T cells. Further, CD8+ T cells exhibited more suppressed phenotypes in the mornings and more antitumor phenotypes in the evenings. Based on this, the researchers developed “morning” and “evening” T cell signatures, the latter of which strongly correlated with enhanced survival in TCGA data for patients with melanoma.
In effector CD8+ T cells, genes associated with T cell activation, suppression, migration, and effector functions followed circadian rhythms. Notably, expression of Pdcd1, as well as surface PD-1 oscillated throughout the day, peaking in the morning and troughing in the evening in both effector and overall CD8+ T cell populations. This effect was abrogated by deletion of Bmal1 in T cells, indicating that it follows a circadian rhythm that is T cell-intrinsic. Similar results were observed in synchronized human CD8+ T cells.
Following these observations, the researchers tested the administration of anti-PD-1 at different times of day and found that B16F10-OVA tumors showed reduced growth when anti-PD-1 was administered in the evening versus in the morning. These differences were dependent on CD8+ T cells and BMAL1 in T cells, and were also evident in an MC38 model. When looking at mice treated with several cycles of anti-PD-1, the timing of the first dose had the strongest impact on tumor growth, in contrast to subsequent doses, which showed less significant time-of-day-dependent differences. Administration of the first dose of anti-PD-1 in the evening, but not in the morning, was associated with increased CD8+ T cell activation, IFNγ production, granzyme B production, and degranulation in the tumor. Combining anti-PD-1 with transfer of OT-I cells in the evening further reduced tumor burdens compared to in the morning.
Finally, the researchers analyzed melanoma samples from patients who had undergone surgical removal of their tumors at different times of the day, and found that CD4+ and CD8+ T cell numbers were highly dependent on the time of day, peaking in the early afternoon. They then utilized their murine RNAseq data to develop tools to estimate times of day for both mouse and human samples. Assigning time-of-day predictions to data from 103 patients with melanoma from TCGA showed differences in the ratios of exhausted to non-exhausted T cells at different times of day. The data for patients generally aligned with the data from mice, but phase-shifted to account for their opposite periods of rest and activity. Further, higher expression of the “evening” RNA signature identified in mouse melanoma T cells correlated with improved responses to immune checkpoint blockade in patients.
Overall, these results suggest that endothelial and immune cell-intrinsic circadian rhythms, along with environmental circadian cues alter the tumor microenvironment and the quantity and quality of immune infiltrates depending on the time of day. These results add to the mounting evidence that circadian rhythms can be capitalized on to improve various forms of immunotherapy simply by administering them at the optimal time of day.
Write up and image by Lauren Hitchings




