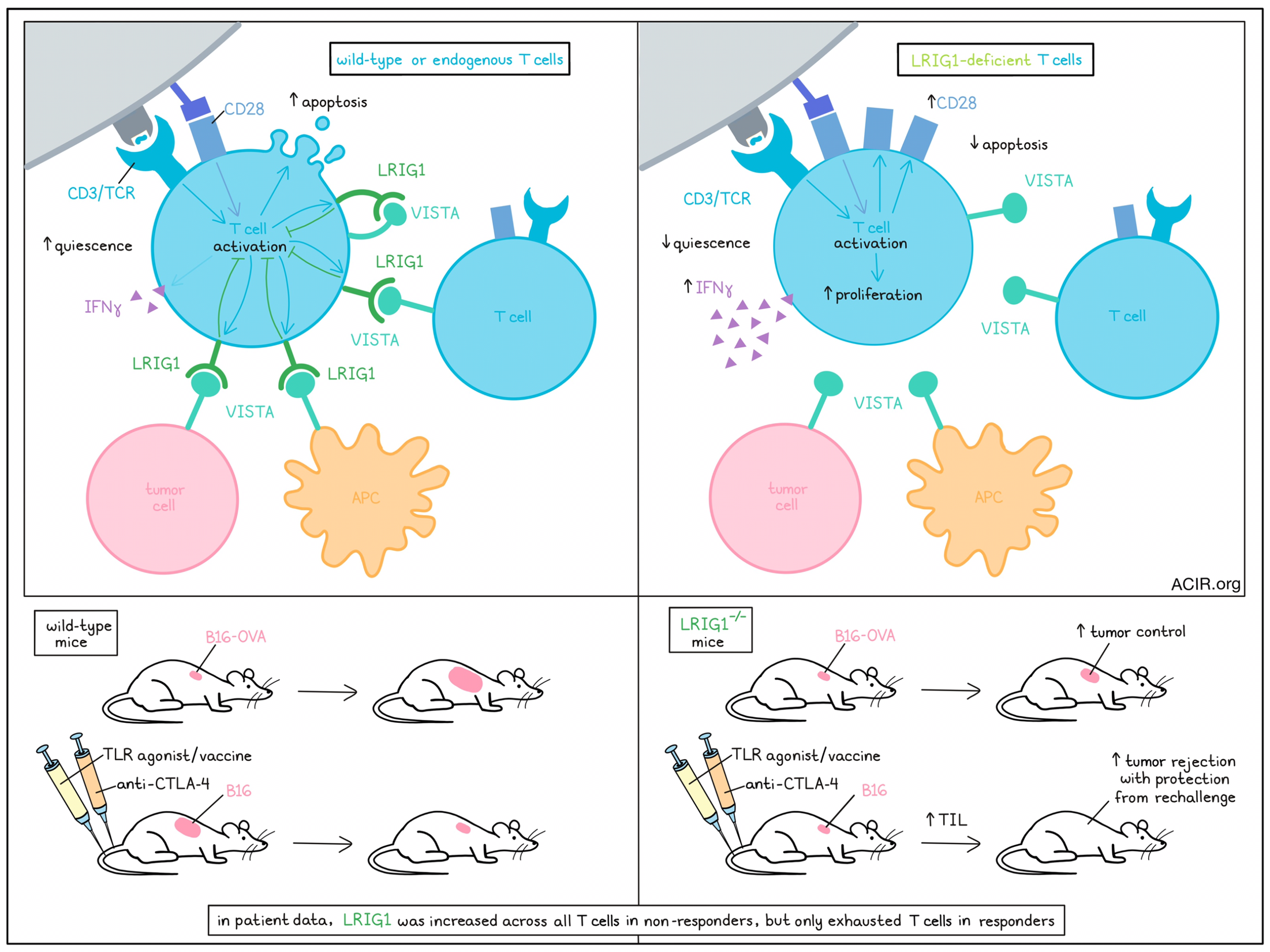
Immune checkpoint inhibitors have revolutionized the cancer immunotherapy field, and V domain immunoglobulin suppressor of T cell activation (VISTA) has garnered attention as a potentially relevant immune regulatory molecule. In preclinical models, VISTA blockade has promoted CD8+ T cell infiltration and inflammatory cytokine secretion in tumors, leading to antitumor efficacy. VISTA appears to function both in cis (between T cells) and in trans (between APCs and T cells), but the cognate receptor(s) are not known. In a recent publication in Science Immunology, Ta et al. identified LRIG1 as an engager of VISTA that controlled antitumor immunity by constraining T cell proliferation and effector function.
Ta et al. took a direct approach for receptor identification by using selective proteomic labeling proximity ligation assay using tyramide (SPPLAT) on wild-type murine splenic T cells or T cells knocked out for VISTA. Leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) was identified as a binder of VISTA, and the result was validated in T cells and HEK293T cells using co-immunoprecipitation and SPPLAT. The binding affinity of the extracellular domains was elucidated by surface plasmon resonance (~1 nM), with minimal variation between neutral and acidic conditions (PSGL-1 had previously been identified as a receptor for VISTA, but only under acidic conditions). Split-luciferase-tagged VISTA and LRIG1 were used to probe the relevance of binding in cis and in trans. Luciferase activity was observed in T cell cultures and in a B16OVA model, suggesting binding partnership between T cells in cis. Furthermore, luciferase activity was observed when LRIG1-labeled T cells were exposed to VISTA-labeled DCs in vitro and tumors in vivo, yet was abrogated in absence of VISTA, suggesting that LRIG1 can also act in trans.
LRIG1 was not detected on naive T cells, yet was induced following activation and increased in response to TCR stimulation. Given that LRIG1 is activation-inducible, the authors sought to understand its impact on T cell activation and signaling. Co-expression of VISTA and LRIG1 in T cells, but neither alone, inhibited phosphorylation of downstream TCR signaling proteins upon stimulation with anti-CD3/CD28 antibodies. Conversely, knockout of LRIG1 (Lrig1-/-) or VISTA (Vsir-/-) enhanced phosphorylation of signaling molecules, suggesting that ligation of VISTA with LRIG1 constrained intracellular TCR signaling. Consequently, activated Lrig1-/- T cells exhibited up to 3-fold greater proliferation and IFNγ secretion compared to wild-type (WT) T cells, along with a significant reduction in apoptosis. Similar trends, including increased expression of the costimulatory molecule CD28, were observed when Lrig1-/- cells were stimulated in an antigen-specific manner by OVA peptide-loaded DCs.
To parse the effects of cis and trans VISTA engagement on T cell phenotype, the authors generated VISTA knockout (Vneg) and VISTA-overexpressing (VOE) DC2.4 cells and cultured them with T cells. In culture with Vneg DCs (where only T cell-derived VISTA was present), Lrig1-/- T cells proliferated more substantially than WT T cells, suggesting that cis engagement plays a role in VISTA/LRIG1-mediated immune suppression. In the presence of VOE DCs, WT T cells failed to expand, while Lrig1-/- T cells expanded vigorously, suggesting remediation of the inhibitory effects of VISTA signaling in trans. Vsir-/- T cells (hence unable to engage T cell LRIG1 in cis) failed to proliferate in culture with VOE DCs, confirming the contribution of trans signaling. To confirm the contributions of cis and trans signaling in the TME, where other putative binding partners of VISTA could be present, VOE and Vneg MC38-OVA tumor cells were generated, and 1:1 mixtures of WT and Lrig1-/- T cells were administered. WT T cells failed to expand in VOE tumors while Lrig1-/- T cell expansion was unaffected, suggesting that trans VISTA signaling plays a role in vivo and that knockout of LRIG1 conferred resistance to this mode of suppression.
Next, the authors sought to examine the therapeutic impact of LRIG1 deletion for cancer immunotherapy. To elucidate the effects of LRIG1 deficiency on TIL proliferation and phenotype in a standard tumor model, WT and Lrig1-/- T cells were adoptively transferred and profiled in B16-OVA tumors. Lrig1-/- T cells robustly expanded after 1 week, while WT T cells steeply declined, leading to a >10-fold difference in accumulation by 2 weeks. Gene set enrichment analysis (GSEA) of TILs indicated enrichment of pathways and genes related to cell cycle regulation in Lrig1-/- cells. Next, tumor growth was compared in WT and Lrig1-/- mice, where antitumor effects would be mediated by endogenous T cells. Lrig1-/- mice experienced significant tumor control compared to WT mice bearing B16-OVA tumors, with TILs upregulating Ki67, IFNγ, and CD28, and being more resistant to cell death. In the non-OVA transfected B16 melanoma model, treatment with a TLR agonist/peptide vaccine furthered tumor control, while addition of CTLA-4 blockade led to 80% rejection and 76% resistance to rechallenge. Lrig1-/- mice exhibited a greater frequency of TILs, including those specific for the tumor antigen TRP2, along with increased CD28 and Ki67 expression and superior IFNγ expression upon peptide restimulation ex vivo.
Single-cell RNA sequencing (scRNAseq) was conducted to further understand the nature of CD8+ TILs and their modulation by LRIG1 deficiency. CD8+ CTLs separated into 12 clusters; 6 clusters expressed Tcf7 (the gene for TCF1), indicating stemness, while the remaining 6 likely represented cells in terminally differentiated states. The C1 cluster, which expressed Tcf7, but lacked both effector genes and inhibitory receptors, was significantly enriched in WT mice compared to Lrig1-/-. This result was validated by flow cytometry and in an adoptive transfer model with Pmel-specific T cells, further indicating that the quiescent state could be induced in activated tumor-specific T cells, and was not merely a consequence of naive T cell infiltration. The reduction in quiescent Lrig1-/- T cells was accompanied by an increase in the frequency of Tpex cells, which exhibited superior responses to peptide stimulation.
To interrogate the relevance of LRIG1 in human cancers, the authors first showed that LRIG1 was induced upon T cell restimulation in patient samples, while VISTA expression was unchanged. They then investigated a melanoma scRNAseq dataset, where a T cell cluster resembling the quiescent C1 cluster in mice was identified. LRIG1 was broadly expressed across T cell clusters in patients that failed to respond to ICB, while expression was more restricted to exhausted cells in responding patients. CD8+ TILs of non-responding patients were enriched for LRIG1, suggesting a role for LRIG1 in the regulation of human T cell effector function.
Overall, these findings identify LRIG1 as a co-inhibitory T cell receptor that functions by binding VISTA. VISTA engaged LRIG1 both in trans and in cis to block TCR signaling pathways, which led to diminished proliferation, CD28 expression, and cytokine secretion. In vivo, LRIG1 knockout greatly enhanced TIL persistence and cytotoxicity, preventing acquisition of a quiescent phenotype and promoting tumor control. LRIG1 expression in human TILs was associated with response to checkpoint blockade, suggesting that blockade of the LRIG1/VISTA axis may be an attractive and practical option for cancer immunotherapy.
Write-up by Morgan Janes, image by Lauren Hitchings
Meet the researcher
This week, senior author Lily Wang answered our questions.

What was the most surprising finding of this study for you?
There are pretty clear phenotypes of the LRIG1 KO T cells when examined in vitro, including better proliferation, better survival, and better cytokine production. Although these were sort of “expected” based on the hypothesis, the most surprising data was from in vivo experiments in murine tumor models: LRIG1 KO mice transiently delayed tumor growth… the long-term tumor-free survival was achieved when in conjunction with a peptide vaccine therapy. This result suggests that we need to continue to explore new combinatorial therapeutic strategies to completely eradicate cancer and improve survival benefit.
What is the outlook?
Outside of T cells, LRIG1 has been studied in cancer cells and stem cells. But for T cell biology, LRIG1 is a newly discovered coinhibitory receptor and a new player in regulating antitumor T cell responses. We are only seeing the tip of the iceberg. Many outstanding questions remain to be addressed. For example: does LRIG1 have other ligands besides VISTA?, what signaling adaptors work with LRIG1 to change T cell function and fate?, is there a role of LRIG1 in other immune cell types?, how is LRIG1 expression is regulated?, and can LRIG1-targeted inhibitors be developed for cancer therapy?.
Like other inhibitory immune checkpoint receptors, the biology of LRIG1 may be relevant to not only cancer immunotherapy, but also for autoimmune and inflammatory disease. LRIG1 antagonists and agonists may be suitable for treating various immune dysfunctions. This study opens a door to an exciting new direction of research in tumor immunology and other diseases with immune dysfunctions.
What was the coolest thing you’ve learned (about) recently outside of work?
VISTA is also a Visible and Infrared Survey Telescope for Astronomy: “VISTA” has been gazing deep into the cosmos for decades. We have been studying the “VISTA” molecule for more than a decade (the initial discovery was made in 2011). I feel that studying VISTA is like a deep gaze into the mystery and wonder of the cosmos of biology.




