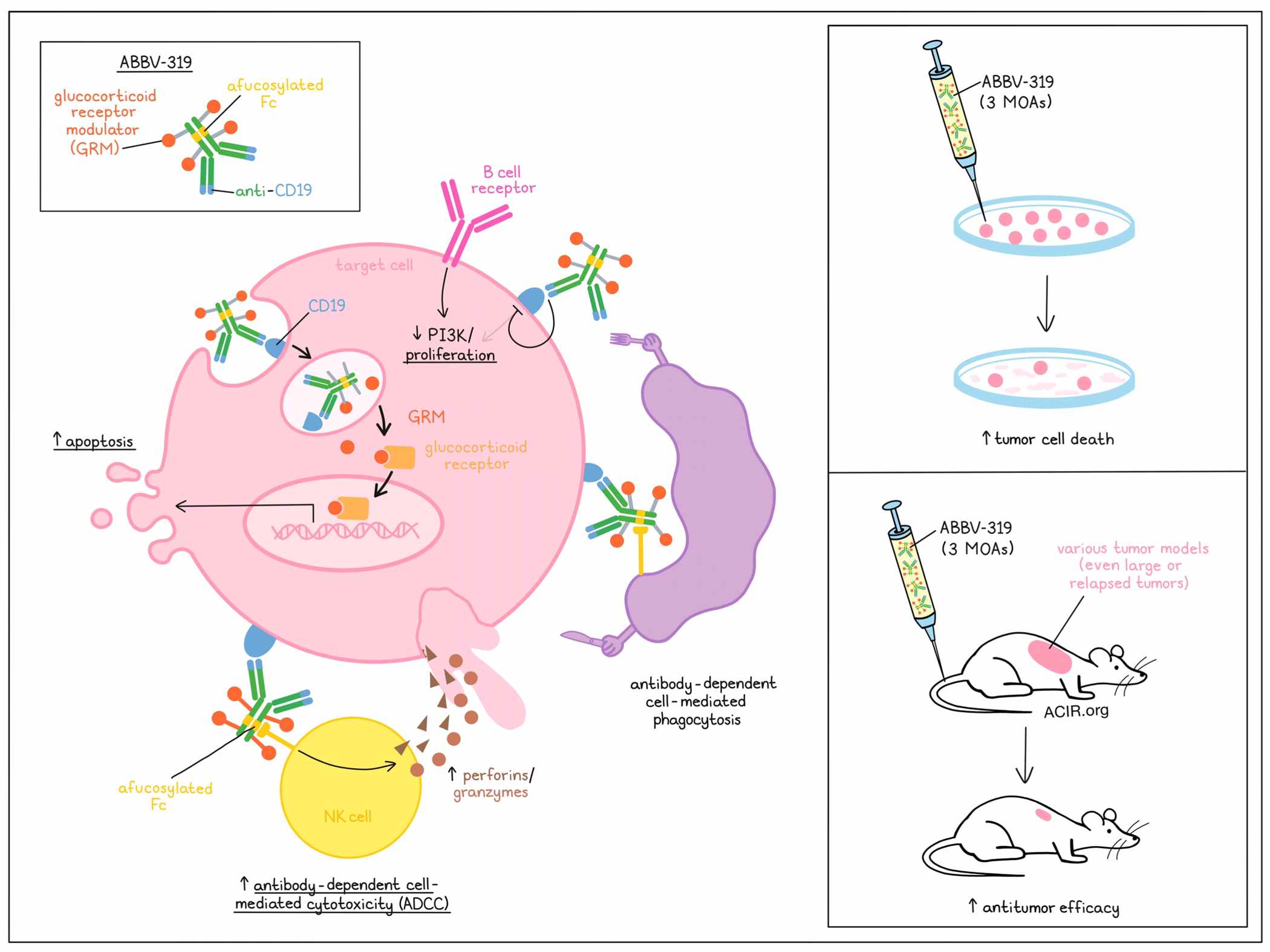
Glucocorticoids are used in combination with chemotherapy and rituximab for the treatment of B cell malignancies, such as diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), and acute lymphoblastic leukemia (ALL). They function as glucocorticoid receptor (GR) agonists to act on downstream signaling related to cell cycle progression and apoptosis genes. However, their clinical potential is limited, as systemic treatment is associated with dose-limiting adverse events. Chang et al. characterized the mechanisms of action of a CD19-targeting agonist GR modulator (GRM) antibody–drug conjugate for the treatment of B-cell malignancies. Their preclinical workup was recently published in Blood.
ABBV-319 has a GRM that is conjugated to an afucosylated CD19 antibody (afCD19 mAb) via an alanine-alanine protease cleavable dipeptide linker. This GRM payload was more potent than the clinical glucocorticoids dexamethasone (25x more potent) and prednisolone (>300x) in vitro when treating glucocorticoid-sensitive B cell malignancy cell lines.
Treating cell lines with ABBV-319 for 24 hours resulted in the internalization of CD19 and lysosomal trafficking. Further, there was a dose-dependent activation of glucocorticoid response elements, confirming that the GRM payload was released in the lysosome. Treatment induced dose-dependent cell killing that was dependent on CD19 expression. Testing a panel of DLBCL, mantle cell lymphoma (MCL), FL, and ALL cell lines, ABBV-319 reduced proliferation, without a significant association between sensitivity to treatment and CD19 or GR expression. Even cell lines with MYC and BCL2 or BCL6 rearrangements were responsive to treatment, which are features that in human disease mark high-grade lymphomas with poor survival outcomes.
RNAseq analysis of treatment-induced changes in the cell lines showed an increase in the expression of GR targets and gene sets related to steroid/corticosteroid response. To assess the expression of an 8-gene glucocorticoid gene signature, PBMCs treated with ABBV-319, af.CD19 mAb, or a control were subjected to CITEseq (cellular indexing of transcriptomes and epitopes by sequencing). The researchers found that treatment for 24 hours increased the signature in the B cell population, while there was minimal activation in other immune populations. These data suggest that there was a specific delivery of the payload to CD19+ B cells, and that the released payload activated GR transcription.
Further assessing the RNAseq data, higher expression of genes related to apoptosis was detected, such as the upregulation of BIM. This increase in BIM correlated with increases in cleaved caspase 3, cleaved PARP, and the sub-G1 population (dead cells). These effects were only detected in responsive cell lines.
Moving to in vivo models, several DLCBL and ALL cell line-derived xenograft (CDX) models responded to ABBV-319, with a single treatment inducing dose-dependent tumor regression, resulting in tumor control. To test efficacy in larger tumors, the RS4;11 model was used, and treatment was initiated when the tumor size reached >600 mm3. A single dose of 10 mg/kg of ABBV-319 resulted in tumor regression for over 40 days, and this effect was better than that of multiple doses of prednisolone at 50 mg/kg or GRM at 10 mg/kg.
Pharmacokinetic analyses showed a dose-dependent increase in the antibody concentration, suggesting linear pharmacokinetics in mice. This increase in the antibody serum concentration correlated with the antitumor effects.
In patient-derived xenograft (PDX) DLBCL models, using tumors that were treatment-naive or relapsed after 4-7 rounds of the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen, a single dose of 10 mg/kg of ABBV-319 resulted in tumor regression, with tumor growth inhibition seen in 10/10 PDXs, and regression in 9/10 PDXs. They also observed efficacy in 7/10 PDXs when the tumors had reached a size of 1000 mm3 before treatment.
To determine the mechanism of action, the researchers assessed antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). ADCP was observed after treatment, but not CDC in in vitro experiments with various cell lines. The CD19 antibody was afucosylated with the idea of increasing Fc-mediated effector function. When compared to the fucosylated counterpart, ABBV-319 induced more specific lysis of tumor cells when cocultured with PBMCs, and it activated NFAT reporter activity in Jurkat reporter cells expressing high-affinity and low-affinity FxγRIIIa. Additionally, ABBV-319 treatment of various cell lines co-cultured with PBMCs resulted in specific lysis of B-cell lymphoma cells, suggestive of ADCC. The effects were similar for ABBV-319 and af.CD19 mAb, which suggests that the payload did not negatively impact ADCC effects.
To further assess ADCC, CDXs were grown in a humanized mouse model engineered to express human IL-15, enabling the differentiation of functional human NK cells after CD34+ hematopoietic stem cell engraftment. In this model, a single dose of 5 mg/kg ABBV-319 induced more tumor growth inhibition and durable antitumor responses than it did in CB17 SCID mice. The treatment depleted normal human B cells, but not NK or T cells, and the depletion of healthy human B cells was transient, rebounding over time. Therefore, ABBV-319 was superior to Af.CD19 mAb in this model when dosed at the same level.
Together, these studies into the mode of action of ABBV-319 reveal therapeutic effects in various models for B cell malignancies. The therapeutic effects are induced in three ways; the delivery of the payload induces apoptosis, downstream signaling of CD19 is inhibited, and ADCC activity is enhanced due to afucosylation of the antibody. ABBV-319 is currently being tested in a Phase 1 clinical trial to determine its safety, tolerability, and activity in patients with relapsed or refractory DLBCL, FL, or CLL.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, Anderson Chang answered our questions.

What was the most surprising finding of this study for you?
One of the surprising findings (in a positive way) from our study was that ABBV-319 displayed antitumor activity in all of the DLBCL PDX models (10/10) that we have tested, including both GCB and non-GCB DLBCL subtypes. We were highly encouraged to see robust efficacy in multiple PDX models that were developed from patients refractory to R-CHOP, suggesting clinical activity may be achievable in relapsed/refractory settings. Moreover, ABBV-319 consistently showed superior antitumor activity compared to an unconjugated afucosylated CD19 antibody, suggesting a potential differentiation from a CD19-targeting antibody-based therapy.
What is the outlook?
ABBV-319 is currently being evaluated in Phase I clinical trial (NCT05512390), where its safety, preliminary antitumor activity, and recommended Phase 2 dose will be determined. Given ABBV-319’s novel mechanisms of action (MOAs), we are also evaluating pharmacodynamic biomarkers for each MOA to better understand the relative contribution of the distinct mechanisms to the activity of the molecule. Our ultimate goal is to bring medicines to patients with unmet medical needs, and the ongoing clinical trial will help informing the safety and effectiveness of this new drug and its clinical potential.
What was the coolest thing you’ve learned (about) recently outside of work?
Our group meeting has a "tell me something" session, in which team members take turns to talk about something unrelated to science. A team member presented a high school project that his boys are involved in called VEX Robotic Competition. It was eye-opening to see high school students building robots and compete in a soccer-like tournament. Likewise, I wish there were competitions that would expose high school students to biotechnology early in their career so we can develop the next generation of scientists in medicine and biotechnology.




