Whole tumor vaccines are appealing as immunotherapies, as they contain the complete range of potential tumor antigens. However, tumor cell inactivation methods often degrade important tumor cell structures and antigens, and the immunogenicity of whole tumor cell vaccines is limited. To address these issues, Gao, Luo, Kwong, and Liu et al. developed an intracellular hydrogelation method to inactivate whole tumor cells while keeping cellular structures and antigens intact. Their results from investigating and optimizing such vaccines based on this hydrogelation strategy were recently published in Cell Reports Medicine.
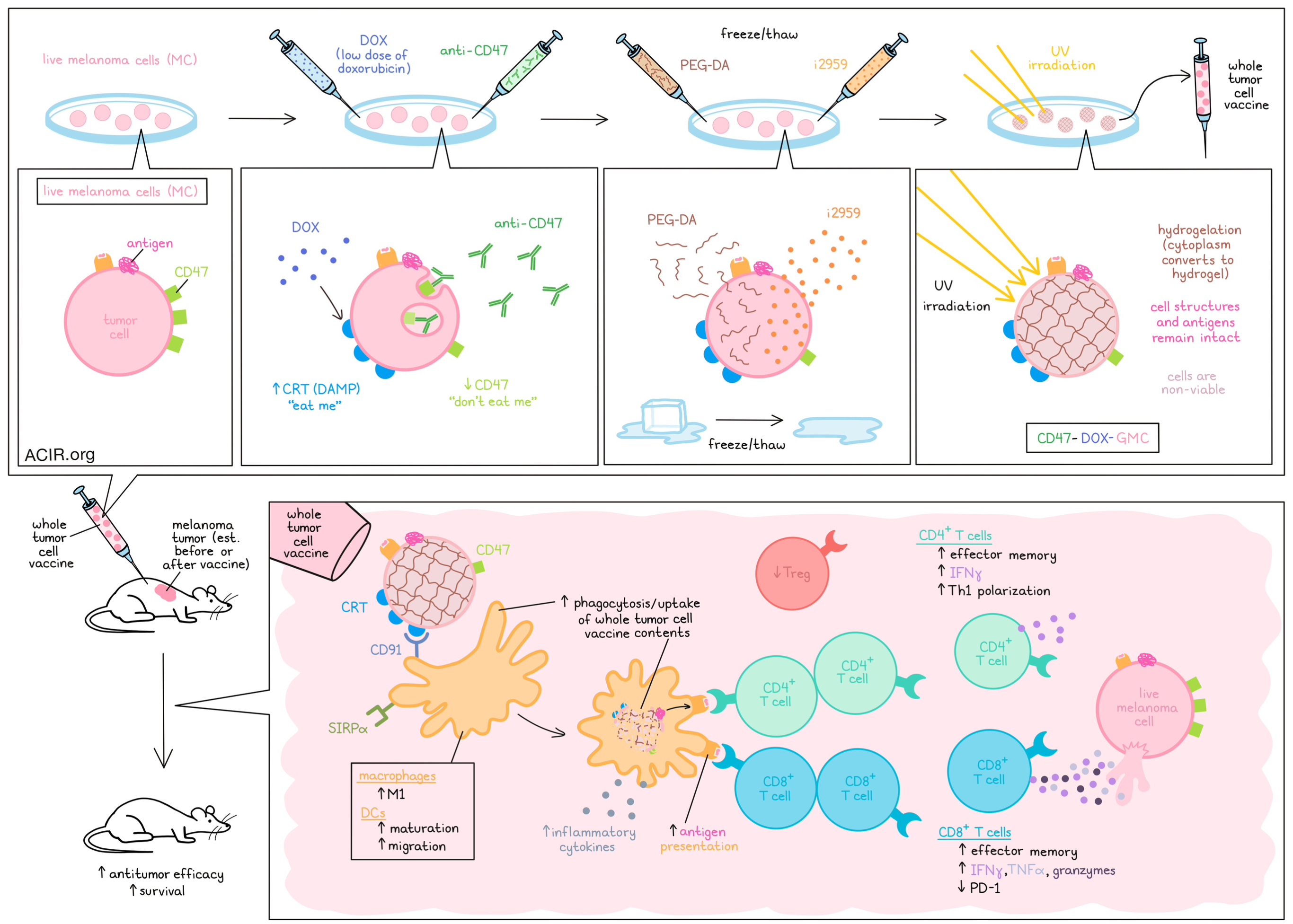
To begin, the researchers selected the gelatin precursor PEG-DA and the low-molecular-weight photoinitiator i2959 to be infused under a freeze/thaw cycle of whole melanoma cells (MCs). Upon UV irradiation, i2959 was activated, inducing free radical polymerization and cross-linking of PEG-DA, converting the cytoplasm into a hydrogel (GMC). Compared to normal freeze–thaw cycles, which leave cells as little more than debris, this hydrogelation strategy maintained the membrane structures and morphologies of the tumor cells, as well as protein structures, including tumor antigens. These cells showed no proliferation in vitro or in vivo, and were confirmed to be non-viable, suggesting that they could be safely utilized for whole tumor cell vaccination.
To increase the immunogenicity of GMC, Gao, Luo, Kwong, and Liu et al. pre-treated live melanoma cells with a low dose of doxorubicin (DOX) that induced growth inhibition, without damaging cells too much to be used as whole tumor vaccines. At the selected dose, DOX induced some immunogenic cell death and increased cell membrane aggregation of calreticulin (CRT) – a damage-associated molecular pattern (DAMP) that engages with CD91 on DCs and acts as an “eat me” signal. Compared to GMCs, DOX-GMCs were phagocytosed by DCs in vitro at a higher rate, and matured the DCs, as evidenced by an increased percentage of CD80+CD11c+ DCs. They also enhanced the expression of molecules involved in antigen presentation and migration, further suggesting increased DC maturation.
To study DOX-GMC as a whole tumor cell vaccine, mice were dosed intraperitoneally with the vaccine, and then challenged with live tumor cells 21 days later. In mice treated with DOX-GMC vaccines, tumors grew more slowly compared to those treated with GMC vaccines or controls, with DOX-GMC increasing survival to 66% and supporting complete rejection of tumors in some animals.
Building on the enhanced immunogenicity of DOX-GMC vaccines, the researchers evaluated the possibility of mitigating immune escape through expression of CD47, which acts as a “don’t eat me” signal on tumor cells. To this end, the researchers added anti-CD47 along with DOX prior to intracellular hydrogelation, generating CD47-DOX-GMC. These cells were phagocytosed by bone marrow-derived macrophages and DCs more readily than DOX-GMC. They also increased the portion of F4/80+CD86+ cells, enhanced production of inflammatory cytokines, increased maturation, and polarized macrophages towards an M1-like state compared to DOX-GMC. Further, immune response-related proteins, including antitumor effectors, were upregulated, suggesting activation of innate and adaptive immune responses.
When CD47-DOX-GMC vaccines were used prophylactically in mice prior to tumor challenge, mouse survival reached 100%, with near complete rejection of tumor challenges. When applied as a therapeutic vaccine against 3-day-old established peritoneal tumors, CD47-DOX-GMC completely eliminated tumors in four of five mice and extended survival, showing increased efficacy over DOX-GMC, GMC, or control treatments.
Having shown that CD47-DOX-GMC increased immune activation of DCs and macrophages through increased uptake of the whole tumor cell contents, the researchers then investigated subsequent effects on adaptive immune responses. Indeed, evaluation of TILs revealed increased proportions of effector memory cells among both CD8+ and CD4+ T cells. Among CD8+ T cells, activation and expression of IFNγ, TNFα, and granzymes were increased, while expression of PD-1 was decreased. Among CD4+ T cells, Th1 polarization and IFNγ expression were increased, while Treg portions were reduced. Similar results were observed in the peritoneal cavity and in the spleen and ascites.
Next, peritoneal CD8+ T cells were isolated from mice that had cleared tumors following treatment with CD47-DOX-GMC. These cells showed strong evidence of cytotoxicity compared to T cells from treatment-naive mice. Further, when adoptively transferred into mice, these CD8+ T cells reduced tumor growth upon challenge. While CD47-DOX-GMC showed limited antitumor efficacy as a therapeutic treatment against 8-day-old established tumors, as did anti-PD-L1, the combination of the two showed enhanced therapeutic efficacy and improved survival rates, yielding better outcomes compared to either treatment alone.
In order to address potential clinical translation issues related to access to tumor tissues, Gao, Luo, Kwong, and Liu et al. collected tumor cells from ascites of tumor-bearing mice. Using this collection, they were able to identify and isolate tumor cells for use as CD47-DOX-GMC vaccines. In mice with 3-day-old tumors, therapeutic treatment with these ascites-derived CD47-DOX-GMC vaccines showed similar antitumor efficacy and survival benefits when compared to vaccines derived directly from tumor cell lines.
Finally, the researchers tested their vaccine models in healthy mice and found that immunotoxicity was limited. Extending beyond melanoma, the researchers developed hydrogelated whole tumor cell vaccines for 4T1 breast cancer cells (CD47-DOX-GBC). In mouse models, these vaccines demonstrated antitumor efficacy, which could be further enhanced in combination with anti-PD-L1.
Overall, these results showed that using Intracellular hydrogelation to inactivate whole tumor cells for vaccine use effectively preserved tumor cell morphology and antigens. When pre-treated with doxorubicin (to induce increase “eat me” signals) and anti-CD47 (to block “don’t eat me” signals) these vaccines showed strong immunogenicity, increasing their uptake by and maturation of antigen-presenting cells, and inducing an adaptive immune response that could eliminate tumors and increase survival. These vaccines also showed synergy with anti-PD-L1, further supporting their clinical translational potential.
Write-up and image by Lauren Hitchings




