CD40 agonists, which promote maturation and activation of antigen-presenting cells (APCs), have consistently shown promise in preclinical research, but have shown limited efficacy in clinical trials, often due to toxicity. To improve the safety and efficacy of CD40 agonists, Osorio, Knorr, and Weitzenfeld et al. developed 2141-V11 – a human IgG1 anti-CD40 agonistic antibody with five-point mutations to optimize its binding affinity to FcγRIIB and enhance cross-linking of CD40 – and evaluated it in a phase 1 clinical trial, the results of which were recently published in Cancer Cell.
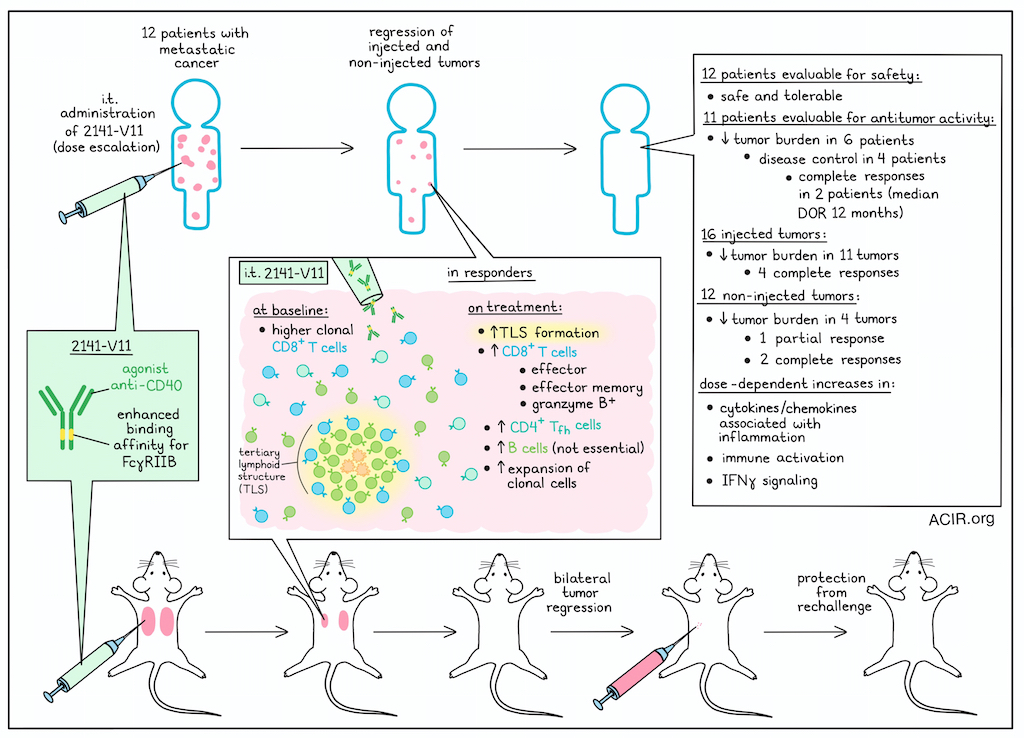
Following prior preclinical research in an immunocompetent mouse model humanized for both CD40 and all human FcγRs (hCD40/hFcγR mice), Osorio, Knorr, and Weitzenfeld et al. established a starting dose and dose escalation regimen for treatment with 2141-V11, and enrolled patients for a phase 1 clinical trial. 12 patients with metastatic cancer who had tumors in the skin, subcutaneous tissue, or lymph nodes that were accessible for intratumoral administration underwent treatment in which 2141-V11 was injected into one or more accessible tumors. Of the 12 patients, 7 had breast cancer, 3 had melanoma, and 2 had renal cell carcinoma.
Following treatment, 10 out of 12 patients experienced adverse events, with 7 experiencing treatment-related adverse events, the most common of which were fever, rigor/chills, and injection-site reactions. These adverse events occurred predominantly at higher dose levels, though none were above grade 3, and no dose-limiting toxicites were observed. Further, while anti-drug antibodies were observed in some patients at higher dose levels, there was no corresponding evidence of serious immunogenicity. Overall, 2141-V11 was safe and well tolerated.
Out of the 12 patients treated with 2141-V11, 11 were evaluable for antitumor activity. A decrease in tumor burden was observed in 6 patients, with 4 patients reaching disease control and 2 of those patients achieving complete responses. Among individual target lesions, decreased tumor burden was observed in 11 out of 16 injected lesions, with 4 injected lesions achieving complete responses and 1 achieving a partial response. Decreased tumor burden was also observed in 4 out of 12 non-injected lesions, with 1 reaching a partial response and 2 reaching a complete response. In line with this evidence of systemic antitumor immunity, pre- and post-treatment peripheral blood samples showed a dose-dependent increase in cytokines and chemokines associated with inflammation, immune cell activation, and IFNγ signaling. For complete responses, the median duration of response was 12 months.
Of the two complete responders, one had melanoma and the other had breast cancer. The patient with melanoma, who was treated in one lesion, had evidence of regression in multiple satellite tumors by cycle 5, which was more pronounced by cycle 10. By cycle 15, the visible lesions had disappeared. The duration of response for this patient was 16.76 months on the study, and for an additional 11 months outside of the study before a tumor recurrence occurred. The patient with breast cancer who achieved a complete response was treated in two locations and had evidence of tumor regression by cycle 2, with resolution of all target lesions in the skin by cycles 4 and 5, along with resolution of non-target axillary and liver metastases by cycles 5 and 7. The duration of response for this patient was 7.1 months, after which recurrence occurred.
Next, the researchers evaluated systemic immune responses among treated patients and found that while total numbers of circulating CD8+ T cells remained largely unchanged, complete responders showed significant expansion of effector, effector memory, and granzyme B-expressing CD8+ T cells, as well as CD4+ follicular helper T cells. Further, complete responders had higher proportions of clonal CD8+ T cells at baseline. These underwent additional proliferation upon treatment, and were enriched in effector states.
Looking at tumor biopsies from the 2 complete responders and one non-responding patient, the researchers noted that infiltration of CD8+ T cells, CD4+ T cells, and CD19+ B cells was increased in responding lesions (both injected and non-injected), which also contained well defined structures consistent with tertiary lymphoid structures (TLSs). In contrast, biopsies from the non-responding patient showed reduced immune infiltration and lack of TLSs.
To further explore the results observed in patients, the researchers returned to their humanized hCD40/hFcγR mouse model, focusing on orthotopic E0771 breast cancer, which was resistant to immune checkpoint blockades and standard agonist anti-CD40. Here, i.t. 2141-V11 treatment induced complete regressions by day 35, with protection from rechallenge 90 days later. In a bilateral tumor model, an abscopal effect was observed. While no TLSs were observed in untreated mice, mice treated with 2141-V11 developed TLSs in most injected (5/6), but not non-injected (0/6) tumors. Injected tumors also displayed higher infiltration of CD8+ T cells and and B cells, but not CD4+ T cells or CD11c+ cells. Similar results were observed after intravesicular administration of 2141-V11 into mice with orthotopic MB49 bladder tumors.
Investigating the immune cells involved in responses, the researchers first evaluated B cells, and found no evidence of class switch recombination. Depletion of CD20+ B cells had no significant effect on the antitumor response, suggesting that they were not essential for antitumor efficacy. Comparing tumors with or without TLSs, the researchers found that in tumors with TLSs, there was significant expansion of CCR7+ DCs, which were enriched for maturation markers (CD40, CD86, and CCR7) and immunoregulatory molecules, consistent with mregDCs. These tumors also showed increased proliferating CD4+ T cells, CD8+ T cells, and effector CD8+ T cells, consistent with enhanced priming. CD8+ T cells also showed clonal overlap between injected TLS-bearing tumors and non-injected tumors, suggesting systemic expansion. Finally, the researchers blocked immune cell egress from tumor-draining lymph nodes (tdLNs) using FTY20, and found that it did not impair tumor rejection of injected tumors, but did abrogate antitumor immunity in non-injected tumors, suggesting that 2141-V11 enhances CD8+ T cell priming within TLS-bearing tumors, while CD8+ T cells that circulated through or were primed within tumor-draining lymph nodes supported antitumor immunity at distant tumor sites.
Overall, the results of this trial support the safety and tolerability of intratumoral 2141-V11 in patients with cancer, and suggest preliminary clinical activity. Further, investigations of blood and tumor samples and relevant mouse models suggest that 2141-V11 supports the formation of TLSs in tumors, enhancing priming and inducing systemic antitumor immunity and durable immune memory. Several ongoing clinical studies are currently evaluating the clinical activity of 2141-V11 in defined tumor types.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, co-first author Juan C. Osorio and lead author Jeffrey V. Ravetch answered our questions.

What was the most surprising finding of this study for you?
While the primary objective of our small clinical study was to evaluate safety and feasibility of intratumoral administration of 2141-V11, we were struck by the impressive antitumor responses observed in a subset of patients. In two participants – treated at two different dose levels – we observed how local injection of the antibody led to complete disappearance of all measurable tumors, including distant, non-injected lesions. This provided the first clinical confirmation of compelling preclinical findings generated over more than a decade in the lab using our humanized mouse models.
Another unexpected discovery was that intratumoral injection not only proved to be safe, but also appeared to trigger the formation of immune niches known as tertiary lymphoid structures (TLS). These structures are thought to promote both local and systemic antitumor responses. While TLS have been associated with improved responses to immunotherapy, to our knowledge, this is among the first therapeutic approaches that seem to actively induce their formation – a finding that could open new avenues for subsequent studies and combination approaches.
What is the outlook?
We are excited to see how this first trial has catalyzed several phase 2 studies nationwide, targeting difficult-to-treat cancers such as malignant gliomas, bladder cancer, and prostate cancer, and early data from these trials are showing promising activity of 2141-V11. That said, not all patients respond, highlighting the need for rational combination strategies to extend the benefit of this therapy. Key questions moving forward include: Which biomarkers predict response to CD40 agonism? What therapeutic partners best synergize with 2141-V11? Are TLS or other specific immune cell-to-cell interactions critical for optimal antitumor activity? Answering these questions will be critical to fully translate this approach into meaningful and durable clinical benefit for patients.
What was the coolest thing you’ve learned (about) recently outside of work?
J.O.: This summer, my weekends in upstate New York with my husband and kids (ages 1 and 3) have been devoted to building a greenhouse from the ground up. It truly takes a village to get it running, and we’ve discovered that manual pollination can be the secret to a happy, abundant harvest.




