In several cancer types, antibiotics limit the efficacy of immune checkpoint blockade (ICB) therapy. On the other hand, metastatic colorectal cancer (CRC) is largely resistant to ICB. The mechanisms behind these effects remain largely unknown. Two recent publications suggest that a novel checkpoint axis, the mucosal addressin cell adhesion molecule 1 (MAdCAM-1)-α4β7 integrin axis, may be responsible for these effects. Feliu, Gomez-Roca, et al. published in Science Immunology on the antimetastatic effects of α4β7+CD8+ T cells and Fidelle, Rauber, Alves Costa Silva, et al. published in Science on how the gut microbiota affects α4β7+CD4+ regulatory Th17 cell tumor infiltration.
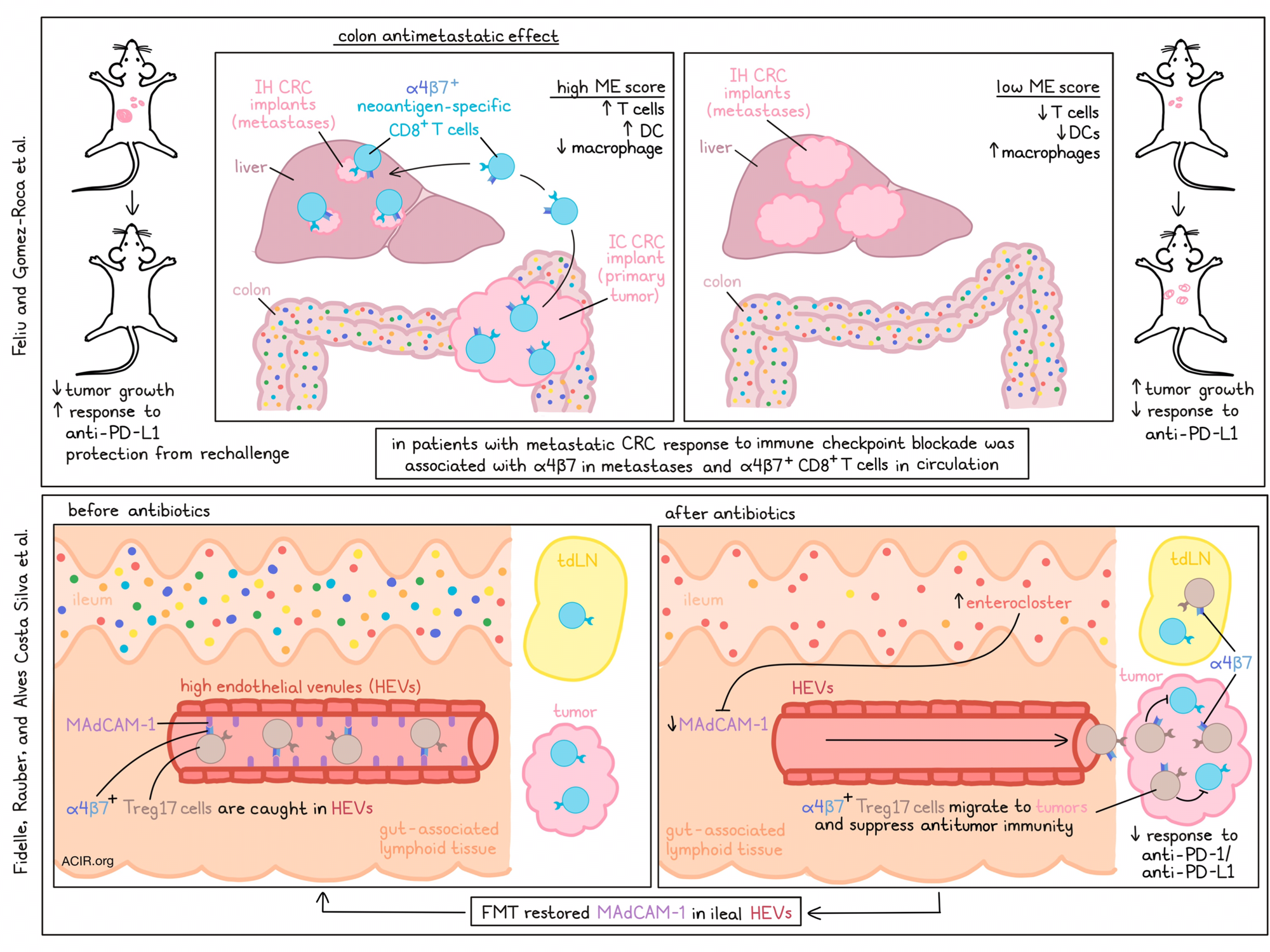
Feliu and Gomez-Roca et al. focused on the effects of this immune checkpoint in metastatic CRC. The researchers implanted mice with MC38 tumors in the liver as a model for one of the most common metastatic sites for colorectal cancer. When mice only received intrahepatic (IH) injection of tumor cells, lethal tumors developed. However, when mice were also injected with MC38 cells into the colon (IC), half of the mice rejected both tumors. This rejection effect was also observed when MC38 cells were injected subcutaneously (SC) and into the colon (IC), while SC alone grew tumors. Additionally, if the IC tumor progressed, the SC tumor did as well, while rejection of IC tumor also resulted in SC tumor rejection.
Based on immune cell infiltration in the IH tumors, mice were segregated into three microenvironment immune (ME) scores, based on levels of infiltrating CD8+ T cells, dendritic cells, and macrophages; low, intermediate, and high. Levels of CD8+ T cells and DCs positively correlated with ME scores, and levels of macrophages inversely correlated with ME scores. Low ME scores were associated with tumor growth. To validate these results, mice were inoculated SC on both flanks and IC. One SC tumor was excised on day 10 to determine the ME score, while the other tumors were assessed for growth. Mice with a low ME score had progression of both tumors, while high ME scores predicted tumor rejection.
The researchers then performed single-cell RNAseq on sorted CD45+ cells from tumors of mice with varying ME scores. Expression of Itga4 and itgb7 genes (encoding the integrin α4β7) was increased in cytotoxic CD8 and cytotoxic NK cell clusters in tumors with high ME scores, while exhausted CD8 and Treg clusters were increased in tumors with low ME scores. Single-cell TCR sequencing revealed increased numbers of expanded clones in IH tumors and blood of mice with IH + IC tumors as compared to mice with only IH tumors. Expanded clones were enriched for Itga4 and Itgb7 expression in IH + IC mice, and these clustered into cytotoxic CD8 clusters. Tracking experiments showed that colon-derived α4β7+CD8+ T cells could migrate to IH tumors. These T cells were activated early after tumor implantation, and in vitro, these cells had increased killing activity. Treating mice with depleting anti-α4β7 antibodies reduced survival, suggesting a key role for this CD8+ T cell subset.
MC38 IH tumors were resistant to anti-PD-L1, while IC tumors responded to therapy. However, the highest efficacy of ICB was seen in mice with IH + IC tumors. Rechallenging of IH + IC mice with MC38 cells IH resulted in tumor rejection. In these mice, high proportions of neoantigen-specific CD8+ T cells were detected. Finally, injection of tumors IH in mice that had previously had IC tumors and were successfully treated with anti-PD-L1 therapy, revealed that mice were protected from IH tumor progression.
To assess the relevance of α4β7+CD8+ T cells in patients’ responses to ICB, metastatic lesions from patients with microsatellite stable (MSS) mCRC treated with ICB combined with targeted agents were assessed using RNAseq. Responders to treatment were more likely to express higher levels of ITGB7 and ITGA4 in metastases. Additionally, a larger proportion of cells expressed α4β7 in pre-treatment circulating CD8+ T cells, and increased levels of circulating α4β7+CD8+ T cells were associated with improved survival. Thus, colon-derived CD8+ T cells marked by high α4β7 expression have important antitumor effects at distant metastatic sites.
Examining a different aspect of gut-derived immune cells, Fidelle, Rauber, Alves Costa Silva, et al. investigated whether bacterial recolonization after antibiotics may be responsible for the lack of ICB efficacy when antibiotics were administered before, but not during, ICB treatment. Treating mice with an antibiotics cocktail resulted in sterilization of the gastrointestinal tract and inhibition of anti-PD-1-induced antitumor effects. Treatment also reduced the expression of Madcam1 (encoding MAdCAM1, the counter-receptor for the integrin α4β7) in high endothelial vessels (HEVs) in the ileum, but not in colon or cancer tissues. After antibiotics treatment, several Enterocloster bacterial species, which have previously been associated with ICB resistance in patients, survived in the ilium. Oral gavage of E. clostridioformis reduced ileal Madcam1 expression, while administration of immunostimulatory Akkermansia or Enterococcus strains increased its expression. Similar effects on inhibition of anti-PD-1-induced antitumor effects were observed when MAdCAM-1 or α4β7 were knocked out or neutralized with an antibody.
In patients treated with antibiotics, expression of MADCAM1 was also reduced in the ileum, but not other colorectal tissues, and a correlation was found between MADCAM1 and IL17A mRNA expression. Additionally, fecal microbial transfer (FMT) from patients with non-small-cell lung cancer (NSCLC) to mice preconditioned with antibiotics downregulated Madcam1 mRNA expression in 3/6 cases, and two of these three samples had an overrepresentation of Enterocloster species.
Tracking experiments in mice 10 days after tumor implant (using Kaede transgenic mice) showed that there was a migration of α4β7+CD4+ T photoconverted cells in the spleen, tumor-draining lymph nodes (TDLNs), and tumor 24 hours after ileal photoconversion of the Kaede fluorescent reporter. Similar results were observed using CSFE labeling of cells into mesenteric lymph nodes (mLNs). RNAseq of these cells revealed overexpression of the Itga4 subunit of α4β7 and of genes involved in Treg function and Th17 polarization. Antibiotic treatment for 14 days resulted in the migration of α4β7+ Treg17 cells from the ileum-draining mesenteric lymph nodes (mLN) to TDLN, but no migration of IL-17A+α4β7+FoxP3-CD4+ conventional T cells or α4β7- Tregs. This migration was also observed in Madcam1-/- mice, and oral gavage of E. clostridioformis could trigger this migration as well.
To determine the clonality of the migrating Tregs, the researchers performed single-cell and deep TCR sequencing. In mice that received E. clostridioformis, there was a higher clonal expansion of Tregs among tumor-infiltrating T cells. The Tregs that reached the tumor changed in phenotype and upregulated genes related to immunosuppression, cytolysis, and type I IFN responses, suggesting that E. clostridioformis increased the proliferative and regulatory potential of migratory Tregs.
Further establishing the role of MAdCAM-1 expression in the gut and the emigration of suppressive T cells into tumors, the researchers found that enforced overexpression of Madcam1 in the liver correlated with Foxp3+ Treg hepatic infiltration. When mice expressed Madcam1 in the liver, the antibiotic-induced resistance to PD-1 therapy was prevented, as Tregs were retained in the liver. These data suggest that MAdCAM-1 acts as an immune checkpoint that controls the retention of Tregs and limits migration to the tumor.
To assess the clinical relevance of these findings, the researchers analyzed this checkpoint in human tumors. Patients treated with antibiotics had lower levels of serum MAdCAM-1 (a known marker of intestine or liver expression of MAdCAM-1), and the baseline serum MAdCAM-1 concentration was a strong positive prognostic factor of survival in patients with NSCLC treated with ICB. Low serum MAdCAM-1 levels could identify patients with PD-L1hi NSCLC tumors not responding to anti-PD-1. Serum MAdCAM-1 was also found to be a biomarker for survival in patients with metastatic renal cell carcinoma or metastatic bladder cancer treated with ICB. Patients with lower serum MAdCAM-1 levels had reduced microbial diversity and a different gut composition, with increased levels of the Enterocloster genus.
The results presented in these two papers suggest a key role for the MAdCAM-1–α4β7 axis in gut antitumor immune responses. MAdCAM-1 is expressed in the gut lining, and activation of colon-derived, tumor-specific α4β7+CD8+T cells can activate anti-metastatic immune responses. However, antibiotics and dysbiosis in the gut can lead to MAdCAM-1 downregulation, resulting in an influx of suppressive Treg populations and resistance to ICB therapy. Therefore, targeting of this pathway may be an interesting new research avenue to balance to improve ICB responses.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, authors of “Distant antimetastatic effect of enterotropic colon cancer-derived α4β7+CD8+ T cells “ Virginie Feliu and Christel Devaud answered our questions.

What was the most surprising finding of this study for you?
In this study, we used an original model of metastatic colorectal cancer (mCRC) that we developed in the lab. We implanted orthotopically syngeneic colon tumor cells in the colon as the primary tumor site, and simultaneously, in the liver as the main metastatic site. Surprisingly, we observed a spontaneous rejection of the primary colon tumors. This initial finding was intriguing, as spontaneous rejection of tumors in mice, without any treatment, had never been observed before. Even more surprisingly, we found that this “tumor rejection phenotype” in the colon could be transmitted to distant and extra-intestinal metastases, such as the liver. Our study then dissected the mechanisms behind this antimetastatic effect off the colon. We identified that gut-derived CD8 T cells expressing alpha4 beta7 integrin were the key components of the antimetastatic effect. Indeed, they are activated locally by colon tumors, migrate to liver metastases, and control tumor development.
What is the outlook?
We want to better delineate the phenotype and functions of these intestinal alpha4 beta7+ CD8+ T cells in the metastases immune response. We also want to understand mechanisms behind the kinetics and magnitude of their “exodus” from the colon to extra-intestinal sites. We are confident that tremendous clinical perspectives may arise from the continuation of this project, in particular for the development of new immunotherapy modalities. First, we may become able to select mCRC patients prone to respond to immunotherapy by using circulating or metastases-infiltrating enterotropic CD8+ T cells as a predictive biomarker of a “favorable antitumor intestinal immunity”. Then, in non-responder patients, we aim to develop new strategies, such as vaccines, in order to specifically stimulate antimetastatic intestinal CD8+ T cells and restore an effective immune response in mCRC.
What was the coolest thing you’ve learned (about) recently outside of work?
VF: A few months ago, I had the opportunity to go to New York City for the CICON 2022, a congress of onco-immunology. Since forever, I have been a big fan of basketball, in particular the NBA. So, I took this chance to attend a Brooklyn team’s NBA game. It was an incredible experience for me, filled with lots of emotion.
CD: Last year, because the COVID crisis went hard on us, as it did for everybody, we finally materialized a project we had a long time ago with my husband – we bought a California van. Since then, we have been enjoying so many nice getaways during weekends and holiday breaks with the two little ones. We are so happy we made this decision. We feel we are gaining so much freedom, having exceptional moments, and discovering so many beautiful landscapes and spots of the French country.
The authors of “A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers” Marine Fidelle, Conrad Rauber, and Carolina Alves Costa Silva answered our questions as well.

What was the most surprising finding of this study for you?
MF: It was very unexpected and exciting to discover by culturomics that antibiotic-induced dysbiosis in mice led to the emergence of Enterocloster spp in the intestine. As soon as we discovered this, it rang a bell and reminded us that these same bacteria were accumulating in patients unresponsive to immunotherapy. This is why we studied the impact of Enterocloster spp. on MAdCAM-1, and those results were corroborated in patients with soluble MAdCAM-1!
CR: It was unexpected the extent to which T cells recirculate from the mesenteric lymph nodes and could be tracked to the tumor microenvironment (TME). MAdCAM-1 and α4β7 as a proxy for these cells was well correlated with mesenteric-derived cells. It was very surprising that these cells were strongly immunosuppressive.
CACS: As a clinician, it was exciting to corroborate the preclinical data in different cohorts of patients with cancer. Behind the figures, there is a huge effort to contact investigators from clinical trials, convince them to embark on our project, write MTAs, and transfer the samples. I have worked on the clinical database, integrating longitudinal clinical and metagenomics data since my first day in the lab together with Dr Derosa. After the saga of culturomics in mice samples, I remembered seeing the results showing again the Enterocloster spp associated with antibiotics intake. So nice!
What is the outlook?
CACS: Nowadays, no consensus exists on a gut fingerprint predicting resistance to immunotherapy, and we lack a diagnostic tool for clinical routine use to select patients and donors to microbiota-centered interventions.
MF: Thanks to this work, we are ready to validate the use of the dosage of sMAdCAM-1 as a biomarker of dysbiosis, associated with poorer outcomes in patients treated with immune checkpoint blockers. Dr Lisa Derosa, Immuno-oncologist at Gustave Roussy, is leading a medical-scientific program named “ClinicObiome, the Clinic of Microbiome”, to offer dysbiotic patients to take advantage of microbiota-centered interventions, including fecal microbiota transplantation, diet and probiotics (https://www.gustaveroussy.fr/fr/clinicobiome).
CR: MAdCAM-1 is a promising biomarker for screening for patients that might profit from fecal microbiota interventions. However, we have to be very cautious to draw wider conclusions at this time, and have to await prospective studies to confirm our findings.
In the long term, patients with a gut microbiome unfavorable to cancer immunotherapy could be treated with fecal transplants from healthy donors to restore balance.
A follow-up project that will implement this clinical approach is underway at University Hospital Heidelberg and will start to recruit patients with hepatocellular carcinoma, hopefully January next year, to receive additional fecal microbiota transplantation to Atezolizumab/Bevacizumab in first-line therapy to improve immune response against tumors. We hope this low-risk procedure for patients will improve the long-term effectiveness of cancer treatment. Concurrently, we are validating α4β7/MAdCAM-1 as a biomarker for response to immunotherapy in a large prospective cohort of gastrointestinal tumors.
What was the coolest thing you’ve learned (about) recently outside of work?
MF: I learned that a flower could charm male bees! It is the Ophrys bee, "Ophrys apifera", whose lip mimics the body of a female bee. These orchid flower forms have diversified due to changes in the regulation of genes activated during petal formation. It is fascinating to see how nature (and the microbiota) find strategies to adapt to their environment.
CR: I have learned with my son that milk teeth that are completely knocked into the jaw in an accident can grow out again in a healthy way. Children are miracles.
CACS: I am learning to surf, which became the best method for me to disconnect and recharge. Recently, I could do the first steps on my longboard, and started surf skating. Like this, I get to “surf” even in Paris!




