In a quest to find a highly specific, off-the-shelf cancer immunotherapy, Chang et al. designed and analyzed Pr20, a T cell receptor mimic (TCRm) antibody targeting HLA-A2 complexed with an epitope derived from the tumor-associated antigen PRAME, and presented proof of concept that TCRm antibodies have therapeutic potential in several human cancers.
PRAME (preferentially expressed antigen in melanoma) is a cancer-testis antigen whose expression in healthy tissue is limited to testes, ovaries, and endometrium, making it an attractive target for immunotherapy. It is overexpressed in many cancers, such as primary and metastatic melanoma, neuroblastoma, hematological cancers (e.g., leukemias), and some breast cancers. However, it is an intracellular protein that is currently inaccessible by regular monoclonal antibodies that target cell-surface proteins.
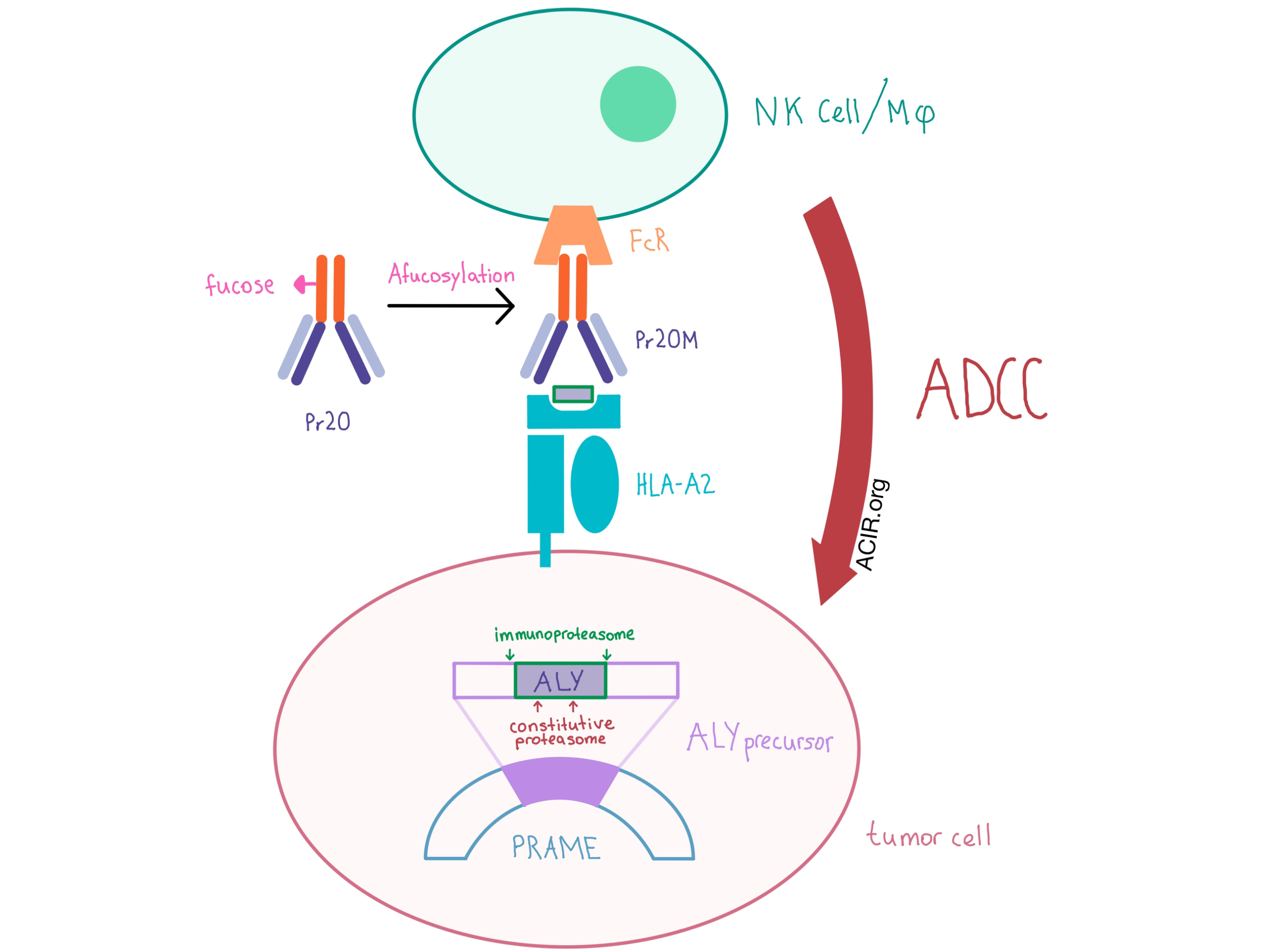
PRAME undergoes proteasomal processing within the cell, and one of its 10-mer peptides (“ALY”) is presented on the cell surface via a complex with HLA-A2. The team designed Pr20, a human IgG1 TCRm antibody, to specifically bind the ALY/HLA-A2 complex, and demonstrated specificity using T2 cells pulsed with ALY. The researchers demonstrated that Pr20 bound the ALY/HLA-A2 complex, but not HLA-A2 alone or HLA-A2 complexed with an irrelevant peptide. Pr20 also bound endogenous ALY/HLA-A2 on various tumor cell lines, including PRAME+HLA-A2+ leukemias and lymphomas, but, as one indicator of safety, no binding was detected to any of the tested immune cell subsets in peripheral blood.
In order to increase the effector function of the TCRm antibody, the team created Pr20M - a version of the antibody with an afucosylated Fc portion and higher Fc receptor affinity. Pr20M was not directly cytotoxic, but was active in antibody-dependent cellular cytotoxicity (ADCC) assays with healthy human donor PBMC effectors against PRAME+HLA-A2+ leukemia and lymphoma cell lines.
Severely immunodeficient NSG mice xenografted with human PRAME+HLA-A2+ leukemia were treated with Pr20M to demonstrate anti-tumor activity of Pr20M in vivo. Analysis with bioluminescent imaging demonstrated that Pr20M significantly reduced tumor growth compared with control, and flow cytometry showed that bone marrow tumor burden was also significantly decreased in one of the leukemia models.
Although the results of the in vitro and in vivo experiments were promising, binding of Pr20 did not occur with all tested PRAME+HLA-A2+ tumor types despite readily detectable PRAME mRNA and protein expression, indicating these characteristics are necessary but not sufficient. As antigen presentation by HLA typically involves protein degradation and additional processing steps prior to HLA loading, the authors hypothesized that some aspect of the antigen processing machinery was an additional, necessary component and turned to examine proteasomal processing. They found that the constitutive proteasome led to significant destruction of the ALY epitope peptide via internal cleavages, while the immunoproteasome left the epitope region intact. These biochemical experiments correlated with biological findings that upregulation of the immunoproteasome with IFN-γ in PRAME+ melanoma and colon adenocarcinoma cell lines led to increased presentation of the ALY epitope based on increased Pr20 binding. Tumors that naturally express the immunoproteasome, such as leukemias and lymphomas, may therefore be the ideal targets for Pr20M.
Overall, the specificity and biological activity of this TCR mimetic antibody provide more evidence that targeting the peptide:MHC complex is a useful approach to accessing tumor-associated or tumor-specific but intracellular gene products, extending the opportunities for off-the-shelf products to treat both hematological and solid tumors.
by Anna Scherer




