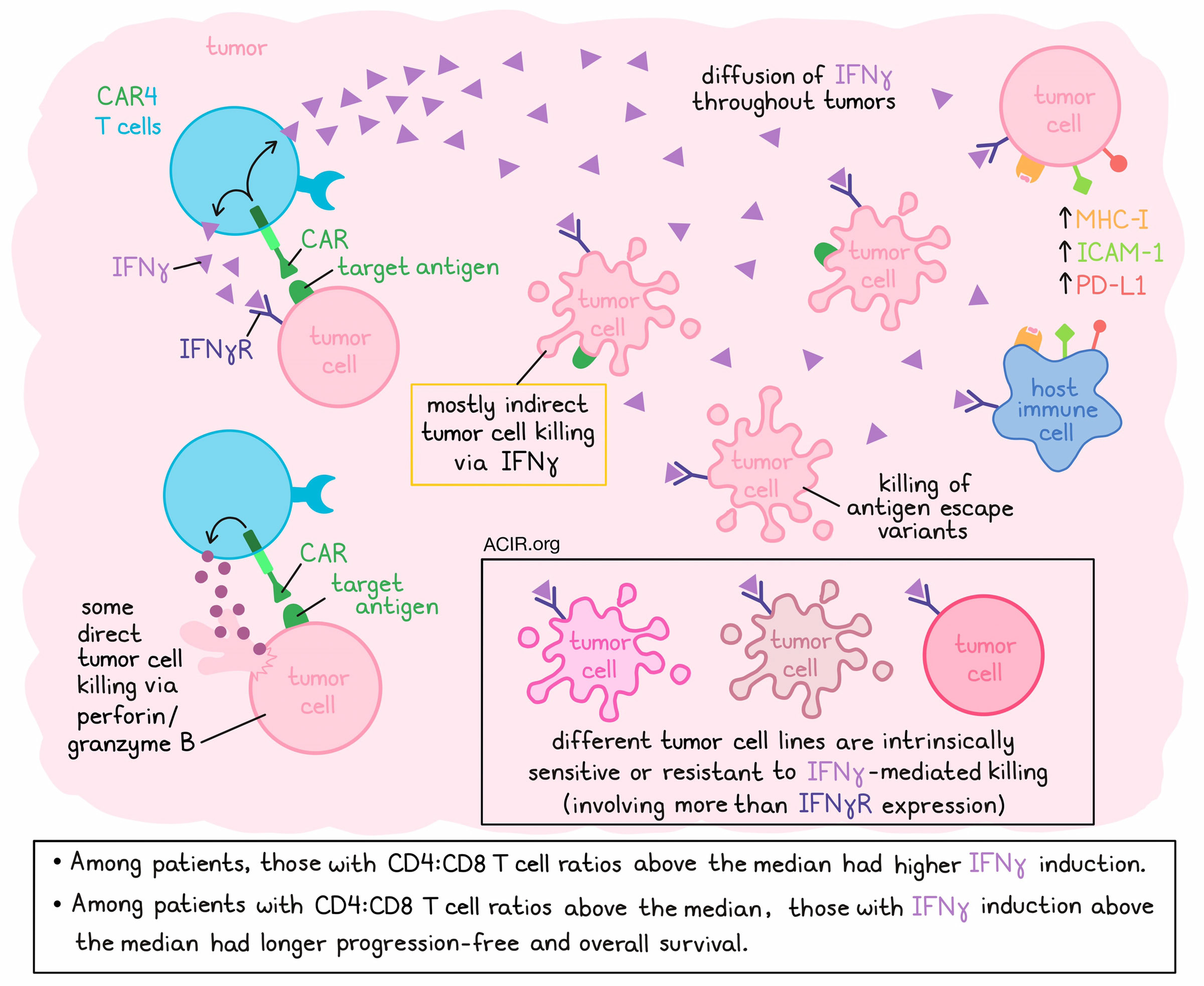
CD8+ T cells often take center stage when it comes to immunotherapy, but in some cases, CD4+ T cells contribute strongly, and may even exert more potent effects than CD8+ T cells. Investigating the variable contributions of CD4+ T cells to antitumor efficacy in CAR T cell therapy, Boulch et al. explored the various functionalities of CAR CD4+ T cells (CAR4 T cells) and found that while they do kill some tumor cells directly through perforin/granzyme B, they primarily induce indirect tumor cell killing through IFNγ. The results were recently published in Nature Cancer.
The researchers began by identifying mouse models that were sensitive (pro-B-cell model) and insensitive (Eμ-myc model) to CAR4 T cell-mediated tumor control. To track tumor cell death, they performed intravital imaging on the bone marrow of mice with tumors expressing a caspase 3 reporter, and as expected, caspase 3 activity/cell death was much higher in the CAR4 T cell-treated pro-B cell model compared to the Eu-myc model.
To parse out how CAR4 T cells induce tumor cell death, mice bearing pro-B-cell tumors were treated with either wild-type (WT) or IFNγ-/- CAR4 T cells. While WT CAR4 T cells mediated tumor control and a survival advantage, these effects were greatly diminished, and the survival advantage was lost when IFNγ production was knocked out. Intravital imaging confirmed that IFNγ-/- CAR4 T induced lower levels of caspase 3 activity in tumor cells.
Next, the researchers used intravital imaging again to classify tumor cell killing events as direct or indirect, depending on whether they occurred with or without detectable CAR T cell contact. Interestingly, less than a third of CAR4 T cell-induced cell deaths were associated with direct contact, while the majority of deaths were indirect. The majority of indirect cell killing (and some direct cell killing) was lost with the use of IFNγ−/− CAR4 T cells, while most direct cell killing was lost with the use of Prf1−/− CAR4 T cells, suggesting that IFNγ mediates killing of tumor cells from a distance, while perforin/granzyme B mediates most killing at close range. Importantly, while loss of perforin/granzyme and direct cell killing had little impact on the efficacy of CAR4 T cells, loss of IFNγ was accompanied by a loss of therapeutic benefit, suggesting that this mechanism of tumor cell killing plays a dominant role in mediating tumor control.
Next, the researchers found that CAR4 T cells, but not IFNγ−/− CAR4 T cells, substantially raised serum IFNγ in WT hosts, and controlled pro-B-cell tumors in IFNγ−/− hosts, suggesting that host-derived IFNγ does not play a major role in the therapeutic activity of CAR4 T cells. Further, while CAR8 T cells could independently control pro-B-cell tumors (mainly via the perforin pathway), they did little to increase IFNγ.
To assess the dynamics of IFNγ diffusion in tumors, the researchers utilized a fluorescent reporter for STAT1 activity, and tracked it using intravital imaging to reveal that both direct injection of recombinant IFNγ and transfer of CAR4 (but not CTRL4) T cells resulted in STAT1 translocation throughout tumors, suggesting efficient cytokine diffusion. At tumor sites, CAR4 T cell therapy led to upregulation of MHC-I, ICAM-1, and PD-L1 (all classically upregulated by IFNs) in both tumor and immune cells at the tumor site.
Investigating exactly how CAR4 T cells and IFNγ mediate long-distance killing, the researchers found that it was not due to indirect effects on other effector cells, nor due to an impact on other host cells, as results were similar across WT, Prf1-/-, and IFNγR1-/- mice. Instead, the effects were found to be due to direct IFNγ-mediated killing, as pro-B-cell tumors underwent apoptosis upon exposure to IFNγ in vitro, and were controlled by direct administration of IFNγ in vivo in both WT and IFNγR1−/− mice. Meanwhile, CAR4 T cells were ineffective against IFNγR1−/− tumors, highlighting the importance of IFNγ sensing by tumor cells for antitumor efficacy. The capacity for long-distance killing was further tested and validated using Transwell assays with both mouse and human cells.
Given the capacity of CAR4 T cells to kill at a distance via IFNγ, Boulch et al. evaluated whether CAR4 T cells could effectively target antigen escape variants. In mixed samples of CD19+ and CD19- pro-B cells, the researchers observed bystander killing of CD19- targets. Similar results were observed with human cells and in a mixed CD19+/CD19- tumor model in vivo.
Different tumors are known to exhibit different sensitivities to IFNγ-induced cell death, and Boulch et al. demonstrated this using several cell lines, including solid tumor lines. Among various pro-B-cell lines (newly generated by viral Abl overexpression), all responded to IFNγ exposure by upregulating MHC-I and PD-L1, but only some showed substantial cell death. When mice were administered tumors containing a mix of fluorescently labeled cells from 3 IFNγ-sensitive and 1 IFNγ-insensitive pro-B-cell line, only the sensitive cell lines were efficiently controlled by CAR4 T cells (but not IFNγ-/- CAR4 T cells), suggesting that their efficacy was highly dependent on tumor sensitivity to IFNγ-mediated killing. Differential tumor sensitivity did not appear to be linked to IFNγR expression, suggesting that other tumor-intrinsic factors likely play a role in determining sensitivity.
Finally, in data from a cohort of 63 patients with diffuse large B-cell lymphoma who were treated with CAR T cells, the researchers monitored CAR4:CAR8 T cell ratios in the blood and IFNγ concentrations in serum. At 1 week post-transfer, around the peak of CAR T cell expansion, CD4:CD8 T cell ratios varied extensively between patients, and patients with high (above median) CAR4:CAR8 T cell ratios exhibited stronger induction of IFNγ. Among patients with high (but not low) CAR4:CAR8 T cell ratios, high IFNγ induction (above the median) was associated with significantly improved progression-free and overall survival.
Overall, Boulch et al. showed that CAR4 T cells primarily exert antitumor effects through production of IFNγ, which can diffuse throughout tumors to induce apoptosis in distant tumor cells that are sensitive to IFNγ-mediated killing. Moving forward, understanding the contributions of these mechanisms and the sensitivity of different tumors to IFNγ could help to predict and/or improve patient responses to CAR4 T cells.
Write-up and image by Lauren Hitchings.
Meet the Researcher
This week, first author Morgane Boulch answered our questions.

What was the most surprising finding of this study for you?
We observed that CD4+ CAR T cells kill tumor cells in an unexpected manner, at distance from tumors, without physically contacting them, but by secreting IFNγ. In the lab, we rely on intravital imaging, a non-biased approach that allows us to explore the mode of action of different immunotherapies in real time. In the past, we have observed that CD8+ CAR T cells, thought to be the main effector cells, engaged into direct cellular contacts with tumors to induce cell death. It turns out to be quite different for CD4+ T cells, and I still remember the day when I first saw tumor apoptotic events popping up at distance from CD4+ CAR T cells.
What is the outlook?
We showed that in lymphoma patients with a high CD4:CD8 CAR T cell ratio, high IFNγ serum level was associated with a better survival. This observation, with the demonstration that, in preclinical models, IFNγ can limit the emergence of antigen escape variants, suggest that it might be helpful to design CAR-based strategies that maximize IFNγ production. In the future, it will also be important to define the optimal CAR T cell product composition (especially the CD4:CD8 ratio) for each individual tumor, in particular, based on its sensitivity to cytokine-induced cell death.
What was the coolest thing you’ve learned (about) recently outside of work?
Two weeks ago, while I was visiting a vineyard and testing wine in Burgundy (France), I found out that French vineyards almost entirely disappeared in the 19th century in France because of a tiny insect called Phylloxera vastatrix. That was until the discovery in the late 1870s that grafting the European fruit-bearing vine variety Vitis vinifera on the root of an American vine, naturally adapted and immune to Phylloxera, protects the sensitive European vine from the Phylloxera attack. Today, almost all European vines are grafted onto American rootstocks. To me, this story is a perfect illustration of innovation, adaptation and collaboration – values shared within the scientific community.




