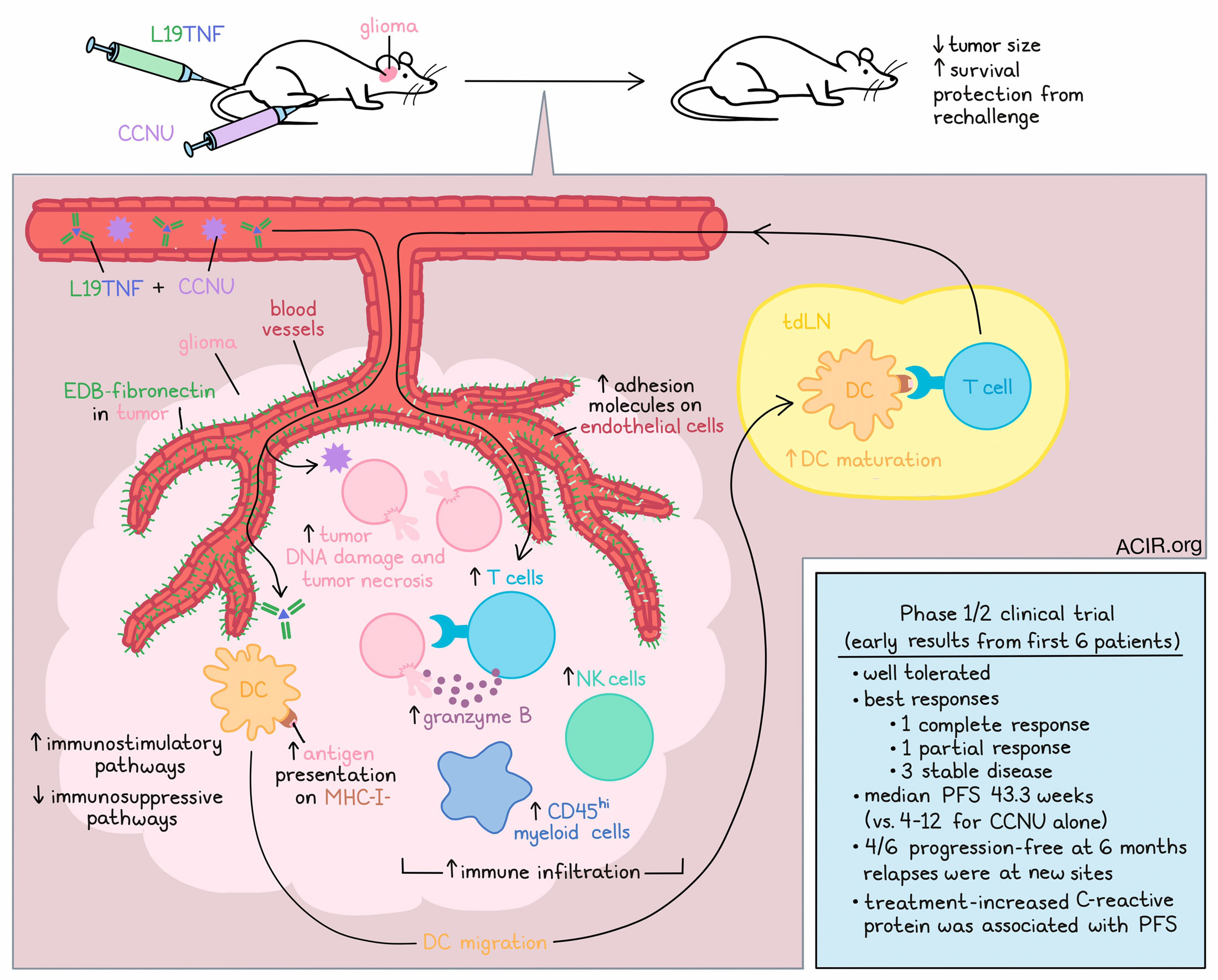
In the hunt for treatments against recurrent glioblastoma, one option that has shown efficacy in mouse models is L19TNF, an antibody–cytokine fusion molecule that targets the EDB domain of fibronectin, which is highly expressed in blood vessels in glioblastoma (but not normal blood vessels), and delivers TNF, which increases the permeability of newly formed tumor blood vessels, induces direct tumor cell killing and tumor necrosis, and activates the immune system. In mouse models of glioma, L19TNF monotherapy prolonged survival and induced tumor necrosis at the cores of tumors, but cells at the edges survived, and tumors regrew. In an effort to expand the antitumor effects of L19TNF, Look and Puca et al. tested it in combination with several approved treatments. Their results, including early results from a phase 1/2 clinical trial, were recently published in Science Translational Medicine.
In immunocompetent mouse models of orthotopic GL-261 glioma, researchers compared responses to L19TNF in combinations with a PD-1 blockade, a VEGF-targeting antibody, or CCNU – a nitrosurea lomustine that acts as an alkylating agent and is typically given to patients with glioblastoma when tumors first progress after surgery. L19TNF plus CCNU emerged as the most effective combination, not only limiting tumor size, but extending survival, with 80% of mice surviving long-term. Similar results were observed in the less immunogenic CT-2A glioma model, where a majority of mice were cured. Further, cured mice were protected from rechallenge 4 months later on the opposite side of the brain.
Investigating this effect in vitro, the researchers found that the combination of L19TNF and CCNU did not exhibit synergy upon direct exposure of tumor cells. Next, the researchers took samples from treated mice at early (1 day after the first administration) and late (1 week after the first of two administrations) time points. At early time points, CCNU (alone or with L19TNF) mediated central tumor necrosis and increased tumor DNA damage, while L19TNF (alone or with CCNU) reduced DCs in tumors and increased them in dLNs, reflecting migration from the tumor to the tdLN. L19TNF also mediated DC maturation. While no difference was observed in T cells or NK cells in tumors at the early time point, they were increased at the late time point, along with an increase in CD45hi myeloid cells. Combination treatment also induced T cells to express more granzyme B both in the tumor core and in the periphery, suggestive of enhanced effector functions.
On the molecular level, L19TNF plus CCNU induced changes in the functionally relevant proteome of tumor endothelial and glioma cells. In fluorescently tagged GL-261, sorted by FACs and analyzed with diaPASEF, the researchers found that L19TNF altered the landscape of endothelial cell-associated proteins related to extracellular matrix organization and cell adhesion processes, including upregulating ICAMs and ST3GAL4, which promote immune recruitment from circulation. Meanwhile, CCNU was found to cause changes in the landscape of glioma cells, including changes associated with tumor cell adhesion and invasion, immune signaling, cell cycle checkpoints, and DNA damage. Combination treatment induced downregulation of proteins associated with immunosuppression and tumor spread, upregulation of the tumor suppressor CDKN2A, and an increase in the expression of chemokines associated with innate and adaptive immunity.
To evaluate the effects of the study agents on antigen expression and presentation, Look and Puca et al. performed immunopeptidome profiling on GL-261 cells before and after treatment. The combination of L19TNF and CCNU had the strongest effect on the induction of H2-Kb and H2-Db molecules and on increasing the number of MHC-I-restricted peptides. Qualitative differences were also observed after combination treatment. Interestingly, the researchers noticed a mutated neoepitope from the WDR1 protein, as well as a retroviral antigen from gp70 that arose after combination treatment. They also observed an increase in RAE-1 – a stress ligand recognized by NK cells. In cocultures of GL-261 cells and T cells, L19TNF with or without CCNU induced the strongest T cell activation, while L19TNF plus CCNU induced the highest T cell mediated lysis of glioma cells. The researchers found that T cells were essential to the antitumor efficacy of combination therapy, as treatment efficacy and protective immunity were lost in mice that lacked T cells or that had been depleted of T cells using antibodies.
Based on the promising results observed in mice, Look and Puca et al. initiated a phase 1/2 clinical trial in patients with glioblastoma at first progression after standard-of-care chemoradiation with temozolomide. The first phase of the trial included 3 dose escalation cohorts, while the second phase of the trial was designed to compare the effects of CCNU monotherapy (standard-of-care) versus CCNU plus L19TNF, with a primary endpoint of overall survival. In early results from the first cohort of phase 1, treatment was well tolerated, and CCNU plus L19TNF has already shown promising results in these first 6 patients. Patient 1, who had multifocal unmethylated MGMT glioblastoma (associated with worse prognosis), exhibited a complete regression of the target lesion in the right front lobe by 9 months, and the response was still ongoing at 18 months, with a 98% reduction of all lesions. Due to COVID-19-related complications, Patient 2 received only one dose of L19TNF, did not receive CCNU, and progressed rapidly upon the cessation of treatment. Patient 3, who had methylated MGMT glioblastoma, experienced a partial response, which is still ongoing, and an 83% reduction of the target lesion at 15 months. Patients 4, 5, and 6 experienced stable disease lasting for several months. These patients relapsed with new lesions at different sites, while the targeted lesions remained stable or shrunk. Compared to the median progression-free survival (PFS) of 4 to 12 weeks reported for CCNU monotherapy, patients treated with CCNU plus L19TNF in this trial had a median PFS of 43.3 weeks, with 4 of the 6 patients progression-free at 6 months. Additional patients are currently being studied.
While white blood cell counts remained stable throughout each treatment cycle, there was a treatment-associated increase in C-reactive protein (a peripheral marker for inflammation), which correlated with progression-free survival. While proper analysis of tumor samples before and after therapy was not possible, recurrent tumor samples obtained after treatment showed a trend toward increased CD8+ T cells, and maintained expression of EDB-fibronectin.
Overall, these preclinical and clinical results show strong promise for the use of L19TNF plus CCNU in the challenging setting of recurrent glioblastoma.
Write-up and image by Lauren Hitchings




