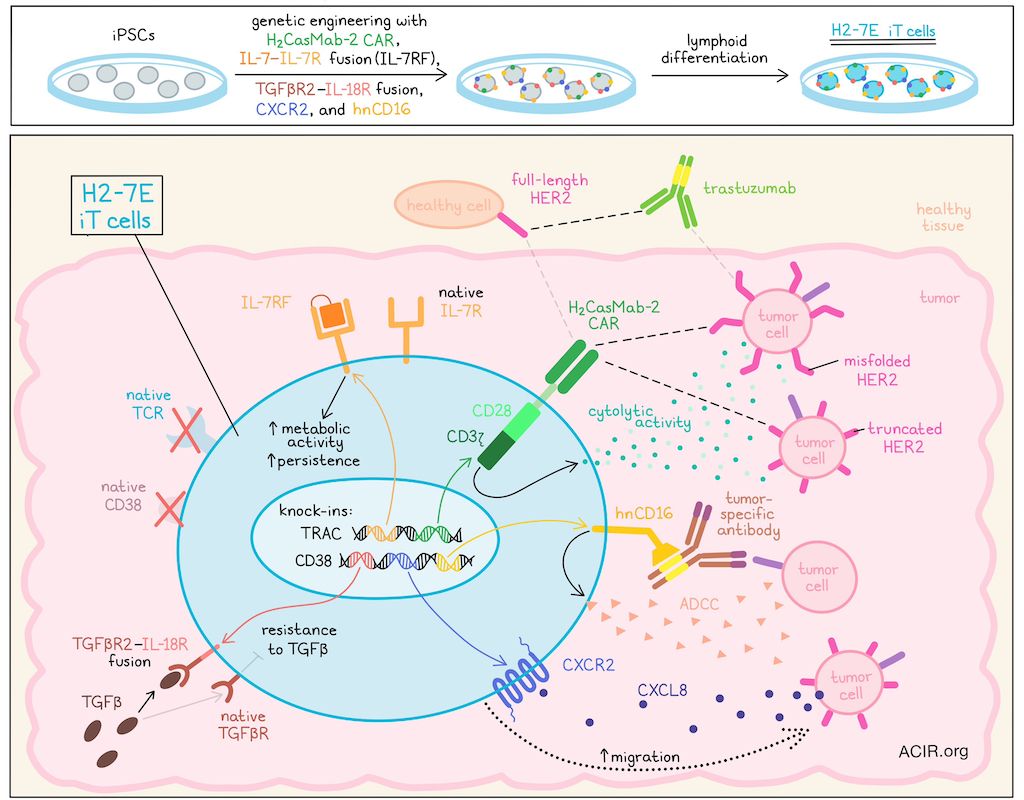
CAR T cell therapies are well known for their potent capacity for killing antigen-expressing target cells. However, they are often hindered by factors in solid tumors and by challenges related to the development of an autologous T cell product. In order to overcome these hurdles and improve CAR T cell therapies, Hosking et al. developed an allogeneic, off-the-shelf CAR T cell product derived from iPSCs, which allowed for extensive genetic engineering and uniform expression of not only the CAR, but also of various transgenes included to enhance antitumor functionalities. This research was recently published in Cell Stem Cell.
To begin, Hosking et al. evaluated HER2-specific antibodies and found that while the commonly used trastuzumab antibody preferentially bound to wild-type/full-length HER2, an H2CasMab-2 antibody additionally, and more preferentially, bound to truncated p95 C-terminal fragments of HER2 and misfolded HER2 variants, which are commonly expressed in cancer cells and may contribute to resistance to trastuzumab. Therefore, the researchers utilized H2CasMab-2 in the design of a second-generation CAR with a CD28 costimulatory domain and a mutated ITAM CD3ζ. Like trastuzumab-based CARs, H2CasMab-2-based CARs demonstrated control of HER2hi and HER2lo cancer cell lines. However, unlike tetrastuzumab-based CARs, H2CasMab-2-based CARs showed minimal targeting of normal cell lines expressing HER2, demonstrating preferential recognition of cancer cells expressing HER2 over normal cells expressing HER2.
Next, the researchers considered ways to enhance CAR T and overcome challenges faced in solid tumors, including heterogeneity and tumor-mediated immunosuppression. In order to extensively engineer cells, Hosking et al. turned to induced-pluripotent stem cells (iPSCs), which allow for precise multi-gene editing, single-clone analysis, and production of a homogeneous effector cell product by selecting for a single clone. Using an iPSC platform, the researchers knocked in (1) the H2CasMab-2-based CAR, (2) an IL-7–IL-7Rα fusion (IL-7RF; to enhance metabolism and persistence), and (3) a TGFβR2–IL-18R fusion receptor (to resist TGFβ-mediated immunosuppression) at the TRAC locus. They also knocked in (4) hnCD16 (to enhance antitumor efficacy through ADCC) and (5) CXCR2 (to enhance T cell trafficking to tumors) at the CD38 locus. Following genetic engineering, iPSCs maintained normal morphologies and genetic stability. After a stage-specific differentiation process to coax the iPSCs into T cells, the resulting cell product, named H2-7E iT, demonstrated commitment to the lymphoid lineage (CD45+CD7+), lacked expression of native TCRs or CD38, and homogeneously expressed the H2CasMab-2-based CAR and other knocked-in transgenes.
Evaluating the HER2-directed cytotoxicity of H2-7E iT cells on tumor targets with varying levels HER2 expression, the researchers found that in co-cultures, H2-7E iT cells were cytolytic against various target cancer cell lines expressing HER2 across a range of levels from high to low. However, compared to trastuzumab-based primary CAR T cells, H2-7E iT cells were less cytolytic against normal cell lines expressing HER2, validating the enhanced specificity of H2CasMab-2-based CAR towards HER2-expressing cancer cells over normal cells. Evaluating antitumor efficacy in vivo, the researchers showed that both HER2hi and HER2lo tumors engrafted into NSG mice could be controlled by adoptive transfer of H2-7E iT cells, though the HER2lo tumors were controlled to a lesser extent. No signs of toxicity were observed.
To take full advantage of the Fc receptor hnCD16 that was engineered into H2-7E iT cells, and to enable multi-antigen targeting, the researchers investigated H2-7E iT cells in combination with either trastuzumab (targeting a separate epitope of HER2) or cetuximab (targeting EGFR). In vitro, combination with either antibody enhanced the cytolytic activity of H2-7E against HER2hi or HER2lo target cells (which also expressed EFGR). This effect was further shown to be dependent on hnCD16 activation of ADCC. In vivo, the researchers compared treatment of tumor-bearing NSG mice with primary H2CasMab-2 CAR T cells, trastuzumab, H2-7E iT cells, or H2-7E iT cells in combination with trastuzumab. While all of the treatments induced some antitumor efficacy, treatment with H2CasMab-2 CAR T cells was variable, and treatment with H2-7E iT cells alone or trastuzumab alone each reduced tumors by about 50%. Meanwhile, the combination of H2-7E iT cells and trastuzumab showed superior antitumor efficacy, leading to rapid, complete, and sustained clearance of tumors in all 8 mice that were evaluated. These results suggested that using antibodies targeting additional antigens enhanced the antitumor efficacy of H2-7E iT cells through hnCD16-mediated ADCC.
Next, the researchers evaluated the functional contributions of the remaining transgene knock-ins – TGFβR2–IL-18R, CXCR2, and IL-7RF – in H2-7E iT cells. In regards to TGFβR2–IL-18R, the researchers found that H2-7E iT cells were indeed more resistant to TGFβ-mediated immunosuppression. For expression of CXCR2, the H2-7E iT cells demonstrated enhanced dose-dependent migration in response to CXCL8 in vitro, and enhanced accumulation in tumors in vivo compared to CXCR2- HER2-targeting CAR iT cells, suggestive of increased trafficking to tumors. Finally, the researchers showed that in the absence of common gamma chain (γc) cytokine stimulation (e.g., IL-2, IL-7, or IL-15), IL-7RF in H2-7E iT cells showed enhanced expression of pSTAT5 relative to IL-7RF- HER2 CAR iT cells, while still retaining native sensitivity to IL-7. Under both stimulation and neglect conditions, a greater proportion of H2-7E iT cells remained viable and showed reduced apoptosis. These results suggested that IL-7RF enhanced the baseline metabolic activity and spare respiratory capacity of H2-7E iT cells, correlating with enhanced persistence and sustained metabolic activity upon tumor challenge.
Overall, these results suggest that extensively edited H2-7E iT cells – derived from iPSCs and engineered to express H2CasMab-2 CARs, TGFβR2–IL-18R fusions, CXCR2, IL-7–IL-7R fusions, and hnCD16 – could serve as a useful allogeneic, off-the-shelf CAR T cell product to preferentially target cancer cells expressing HER2, with the flexibility to co-target additional antigens with antibodies to activate CD16-mediated ADCC. A version of this product is currently being investigated in a phase I clinical trial.
Write-up and image by Lauren Hitchings




