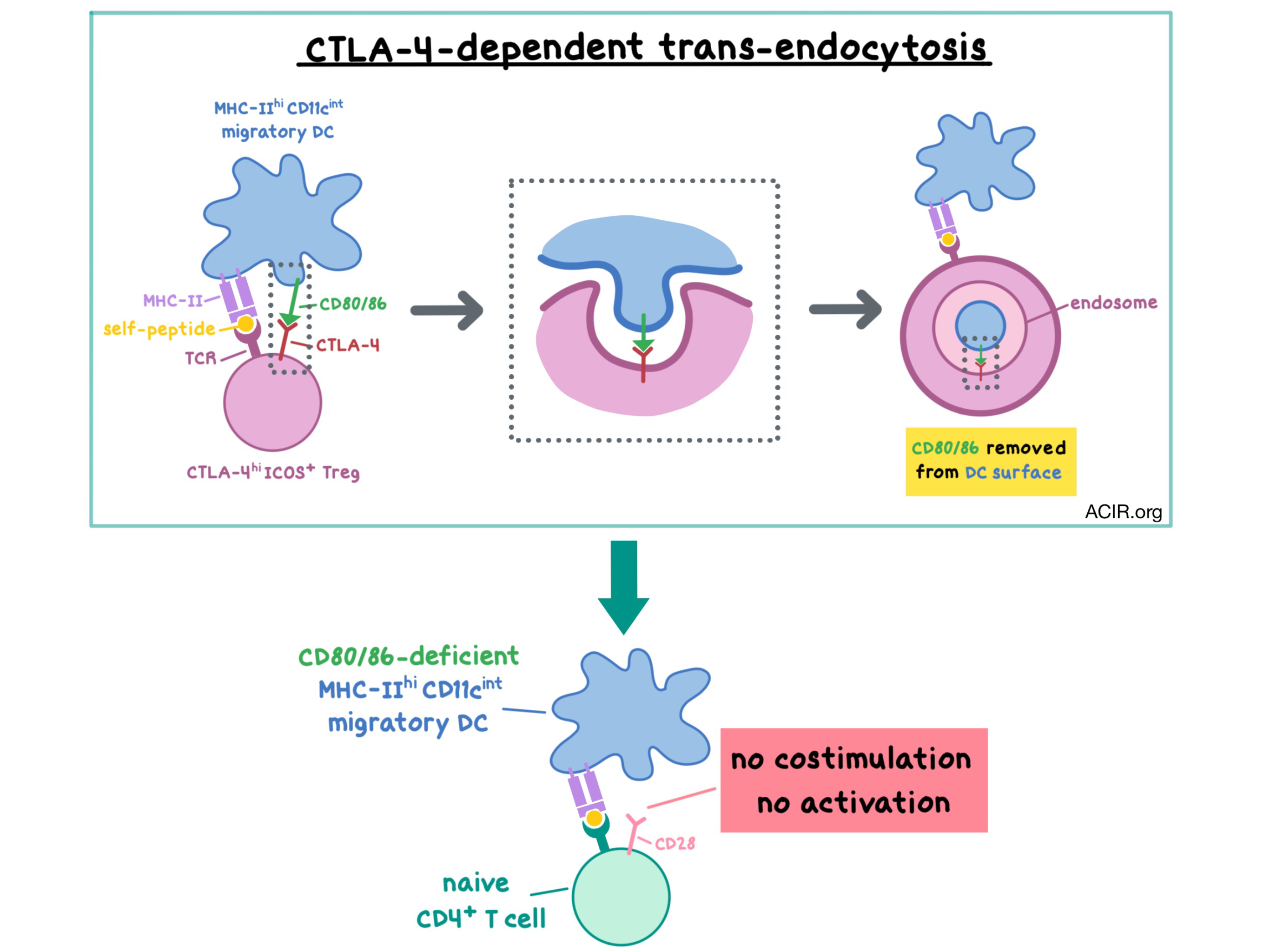
It is well-known that Tregs suppress T cell responses to self-peptides via the CTLA-4 pathway in order to maintain normal immune homeostasis, but the details of this mechanism have not been fully elucidated. In vitro studies have shown that Tregs suppress the CD28-dependent activation of naive T cells by limiting their access to the CTLA-4/CD28 costimulatory ligands (CD80 and CD86). Tregs accomplish this by capturing the ligands from antigen-presenting cells (APCs) via trans-endocytosis (TE). In a paper recently published in Science Immunology, Ovcinnikovs et al. set out to understand the process of TE in vivo and to identify the cell populations that are targeted by Treg-mediated, CTLA-4-based regulation.
The researchers began by assessing the uptake of a fluorescently-labeled anti-CTLA-4 antibody to analyze the cycling and cell surface expression of CTLA-4 in CD4+ T cells in vitro. They found that Tregs constitutively cycled CTLA-4 to the cell surface, while conventional CD4+ T cells (Tconv) required prolonged TCR stimulation to induce higher levels of surface CTLA-4. Using Chinese hamster ovary (CHO) cells that expressed GFP-tagged CD80, the team then demonstrated that Tregs were able to capture and internalize the ligand via TE after 6 hours of stimulation, whereas Tconv cells were able to do the same only after 24 hours of stimulation.
Tregs isolated from the chimeric bone marrow of mice with both wild-type and CTLA-4-deficient cells demonstrated that TE of CD80 was dependent on CTLA-4, was present in effector Tregs and not in resting Tregs, and occurred even at low levels of CD3 stimulation. Using bone marrow-derived DCs and transgenic TCR Tregs, the researchers showed that more CD80 was lost as the amount of peptide stimulation increased in vitro.
For in vivo studies, Ovcinnikovs et al. utilized CD80-GFP-expressing mice that were intravenously injected with CD4+ T cells from DO11 x RIP-mOVA mice (which contained a mix of OVA-specific Tregs and Tconv cells) and immunized with the OVA peptide. Upon an OVA peptide challenge a week later, Tconv cells upregulated CTLA-4 expression but did not exhibit TE. Tregs, however, efficiently trans-endocytosed CD80; specifically, the CTLA-4+ICOS+ Treg population was responsible for the ligand capture. A previous study by the authors showed similar in vivo results for CD86.
Next, the researchers asked whether TE could occur in response to self-antigens. Examination of sites of self-antigen recognition (spleen, lymph nodes, pancreas) in DO11 x RIP-mOVA mice (in which T cells target OVA-expressing β cells in the pancreas, leading to diabetes) revealed much higher CTLA-4 expression on OVA-specific Tregs in the pancreas than at other sites. Once again, Tconv cells showed lower levels of CTLA-4 and the Tregs with high CTLA-4 expression were ICOS-positive. When CD80-GFP-expressing mice with pancreatic expression of OVA (where OVA acted as a tissue self-antigen) were intraperitoneally injected with CD4+ T cells from DO11 x RIP-mOVA mice, OVA-specific Tregs demonstrated CD80 capture in vivo, while Tconv cells did not.
To see if Tregs are capable of TE in response to self-peptides in the absence of exogenous stimulation, the researchers adoptively transferred polyclonal CD4+ T cells into CD80-GFP-expressing mice. Concordant with previous results, Tregs, but not Tconv cells, acquired CD80 in a manner that was dependent on CTLA-4, as this effect was abrogated with an anti-CTLA-4 antibody. As Tregs acquired the GFP-labeled CD80, conventional dendritic cells (cDCs) simultaneously showed a decrease in CD80 staining and in the GFP signal, while no changes in expression of CD80-GFP were observed in macrophages. These results suggest that cDCs are the primary APCs that are targeted by Treg-mediated TE.
Ovcinnikovs et al. then analyzed murine lymph nodes to further ascertain which APCs were targeted by Tregs, and found that abrogating TE via CTLA-4 knockout increased the expression of the costimulatory CD80 and CD86 ligands on MHC-IIhiCD11cint migratory DCs (which traffic self-antigens from peripheral tissues), but did not confer any change in MHC-IIintCD11chi resident DCs or in macrophages; only modest changes were seen in splenic DCs. The changes in migratory DCs were observed in both cDC1 (cross-presenting to CD8+ T cells) and cDC2 (stimulating CD4+ T cells) subsets. Similar results were observed when TE was abrogated via an anti-CTLA-4 antibody. Together, these experiments demonstrated that migratory DCs are the primary target for CD80/CD86 ligand regulation via CTLA-4-dependent, Treg-mediated trans-endocytosis. These findings further improve our understanding of the mechanism by which CTLA-4 and anti-CTLA-4 antibodies regulate immune response.
by Anna Scherer
Meet the Researcher
This week, first author Vitalijs Ovcinnikovs answered our 3 questions.

What prompted you to tackle this research question?
It was during my time studying undergrad Biotechnology at Manchester University when I started to become interested in immunology. I was particularly fascinated with the role that regulatory T cells play in maintaining immune tolerance – how a dedicated cell population protects you throughout life from developing autoimmunity. A huge part of that is CTLA-4, which was known as a key molecule for Treg suppression and a major target for cancer therapy. Yet, there was no consensus on exactly how it works! So, I was very fortunate to meet Professor Lucy Walker, who was moving to the new Institute of Immunity and Transplantation at UCL. At the time, Lucy had just shown that CTLA-4 acts in this bizarre way, by nibbling on its ligands expressed on target cells. As soon as I joined the lab, we proceeded to carefully investigate every aspect of CTLA-4 trans-endocytosis in vitro, but the main question was always to show its effects in a live organism. That alone was a monumental effort to set up. Specifically, showing that changes in costimulatory ligand levels are a result of trans-endocytosis took the better part of my PhD. In the end, however, the toughest question was the most rewarding to finally nail down.
What was the most surprising finding of this study for you?
We were rather staggered to see how rapidly and effectively CTLA-4 can alter costimulation. It does, however, make perfect sense – you are relying on a small population of Treg cells to keep the entire immune system in check. Having seen CTLA-4’s rapid effect in vitro, we expected to see blanket suppression on all antigen-presenting cells in mouse. Surprisingly, that was not the case – migratory dendritic cells stood out as the main target. What makes this population so attractive is yet to be answered, but the fact that they are constantly suppressed under normal conditions certainly implies that migratory DCs may be a key risk factor behind autoimmunity.
Another interesting consequence of our findings is that baseline levels of CD80 and CD86 on certain dendritic cell populations are vastly different in the presence of CTLA-4. This means that you must always keep in mind CTLA-4’s effect whenever you look at DCs in a Treg-sufficient system!
What was the coolest thing you’ve learned (about) recently outside of the lab?
There was this pub outside of the institute, where we spent a fair amount of time. As a side effect I became a serious beer nerd and as soon as I finished my PhD and got my hands on some precious free time, I dived deep into homebrewing. As I discovered, there is a remarkable amount of science in beer! From yeast strain genetics to utilisation of hop compounds to some hardcore chemistry. Brewing, in a way, is very similar to working in a lab. Designing a recipe provides the same excitement as asking a novel scientific question and looking for ways to approach it. So, I often find myself reading papers for my hobby and coming up with experiments I can do on the next batch!




