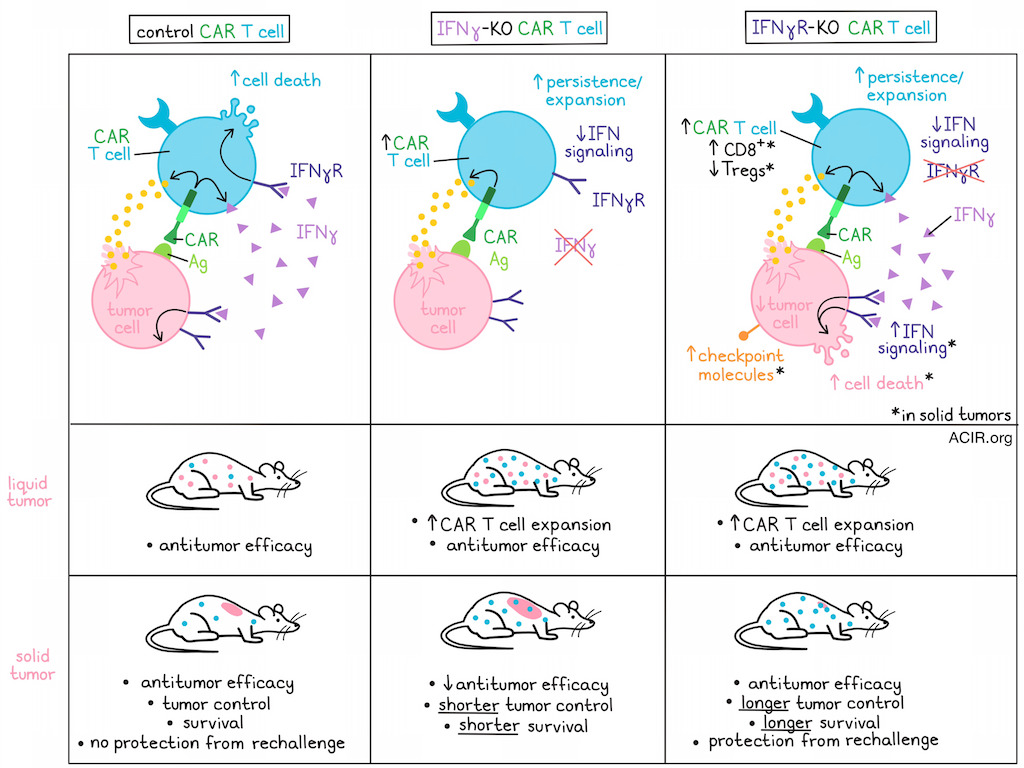
While CAR T cell therapy has shown great success in hematological cancers, relapses may occur, and the therapy has so far shown limited efficacy in solid tumors. Based on their previous data, which showed that genetic deletion of IFNγ in CD19 CAR T cells results in fewer toxicities and better expansion, Bailey, Takei, Escobar, et al. assessed the effects of IFNγ in CAR T cell activity, with the goal of improving CAR T cell efficacy. Their results were recently published in Science Translational Medicine.
To study the effects of IFNγ in CAR T cells, several CD19-targeting CAR-T products were created, including CARCD19 (control), IFNγ-KO CARCD19 (lacking IFNγ production), and IFNγR-KO CARCD19 (cannot respond to IFNγ) T cells. In vitro, activation and cytokine production in response to antigen were similar for all CAR-T, except for IFNγ in the IFNγ-KO CARCD19. However, IFNγR-KO CARCD19 and IFNγ-KO CARCD19 expanded more after antigen exposure. These results were limited to CAR-T with a CD28 costimulatory domain, as in versions with 4-1BB, the loss of IFNγ signaling did not improve expansion.
The three CAR-T versions exhibited similar cytotoxic capacity in vitro. In NSG mice bearing JeKo-1 mantle cell lymphoma, the three CAR-T products induced comparable antitumor activity and survival. However, the IFNγ-KO CARCD19 and IFNγR-KO CARCD19 cells had a higher peripheral expansion after 14 days than the controls.
RNAseq analysis of in vitro-activated CAR-T showed that while all had similar upregulation of highly expressed genes related to antigen exposure, the IFNγ-KO CARCD19 and IFNγR-KO CARCD19 had a decrease in IFN signaling-related genes. Despite the role of IFNγ on checkpoint molecule expression, no differences in checkpoint molecule expression were detected between the three CAR T cell subtypes.
While gene expression suggested better proliferative potential of the IFNγ-KO CARCD19 and the IFNγR-KO CARCD19 cells, in vitro experiments showed similar proliferation. The researchers hypothesized that the increased persistence might be due to protection from IFNγ-induced cell death. Indeed, control CAR T cells upregulated cell death-related genes, increased annexin V, and cleaved caspase 3 after antigen stimulation, and live cell imaging confirmed reduced cell death in IFNγ-KO CARCD19 and IFNγR-KO CARCD19.
Next, the researchers hypothesized that deleting IFNγR in CAR T cells may improve their efficacy in solid tumors. To test this, they developed similar CAR constructs as previously, but targeting mesothelin (CARMESO). As with the CARCD19, all subsets showed similar activation and functionality. The IFNγR-KO CARMESO cells expanded more in response to mesothelin, and both IFNγ-KO CARMESO and IFNγR-KO CARMESO had increased expansion when exposed to mesothelin-expressing pancreatic cancer cell lines in vitro. Similar to the findings with CARCD19, no differences were detected in terms of differentiation, immune checkpoint expression, or proliferation. However, the CAR T cells lacking IFN signaling had reduced apoptotic cell death, suggesting the effects of blocking IFN signaling in CAR T cells (with a CD28 costimulatory domain) were independent of the target antigen.
In vitro, the IFNγ-KO CARMESO had lower antitumor activity, while the IFNγR-KO CARMESO had similar activity as the control CAR-T. The lower activity of the IFNγ-lacking CAR-T may be related to previous findings that IFNγ production by CAR T cells is necessary for antitumor efficacy in solid tumors. Confirming this notion, NSG mice with AsPC-1 pancreatic tumors treated with IFNγ-KO CARMESO had reduced tumor control and survival, while those receiving IFNγR-KO CARMESO exhibited longer tumor control and survival compared to controls, focusing the research efforts on the receptor knockouts.
When the tumor microenvironment was assessed two weeks after CAR-T infusion (IFNγR-KO CARMESO or control), the researchers found that mice that received the IFNγR-KO CARMESO had a 2-fold increase in the CAR T cell population and 50% reduction in tumor cells compared to controls. RNAseq analysis of isolated CAR T cells showed that while the cells had similar in vitro differentiation pre-infusion, the tumor-isolated IFNγR-KO CARMESO exhibited higher levels of CD45RO, GZMA, and GZMB than controls. Among the tumor-isolated IFNγR-KO CARMESO, there were fewer Tregs and more CD8+ T cells than among control CARMESO. Confirming the in vitro data, the IFNγR-KO CARMESO upregulated anti-apoptotic genes, whereas the controls exhibited overexpression of apoptosis- and cell death-related genes. Analysis of the tumor cells showed an increase in IFN-related genes and upregulation of immune checkpoint proteins, tumor suppressor genes, and pro-apoptotic markers in tumors treated with IFNγR-KO CARMESO, suggesting that inhibition of IFNγ uptake by CAR-T drives IFN signaling and cell death in tumor cells.
To determine whether IFNγR-KO CAR T cells could persist and protect from tumor rechallenge, the researchers moved to EGFR-targeting CAR-T, as the tumor model expresses higher levels of EGFR than mesothelin. Mice were treated 2 weeks after tumor inoculation, and control and IFNγR-KO CAREGFR T cells showed similar antitumor activity and mouse survival, with several mice in both groups having complete responses. However, when the cured mice were rechallenged with tumor cells, the ones who originally received control CAR-T were not protected, while those who had received IFNγR-KO CAREGFR had a small tumor relapse that was followed by rapid clearance of the tumor, resulting in long-term survival.
While these results are promising, NSG mice lack an endogenous tumor microenvironment, and IFNγ can affect various cell subsets. Therefore, the researchers assessed if similar results could be obtained in a syngeneic mouse model. T cells from CD45.2+ WT or ifngr-KO C57Bl/6 mice were obtained and transduced to express a B7H3-targeting CAR with a CD28 costimulatory domain. Further, a triple-negative breast cancer cell line overexpressing B7H3 was generated and injected into the mammary fat pads of CD45.1+ C67BL/6 mice. Mice were conditioned with cyclophosphamide, and two days later, infused intravenously with 5x106 CD8+ CAR-T. IFNγR-KO CARB7H3 showed increased antitumor activity and better persistence (measured at day 23) than their WT counterparts.
Together, these data reveal that the deletion of IFNγR from CD28 CAR-T can protect against IFN-induced cell death without affecting efficacy, thereby increasing functional persistence. Further, due to lack of competition for IFNγ, more IFN signaling in tumors aids the antitumor effects of IFNγR-KO CAR T cells in hematological, and importantly, solid tumors in animal models, suggesting this modification in CAR T cell design has potential for further clinical development.
Write-up by Maartje Wouters, image by Lauren Hitchings




