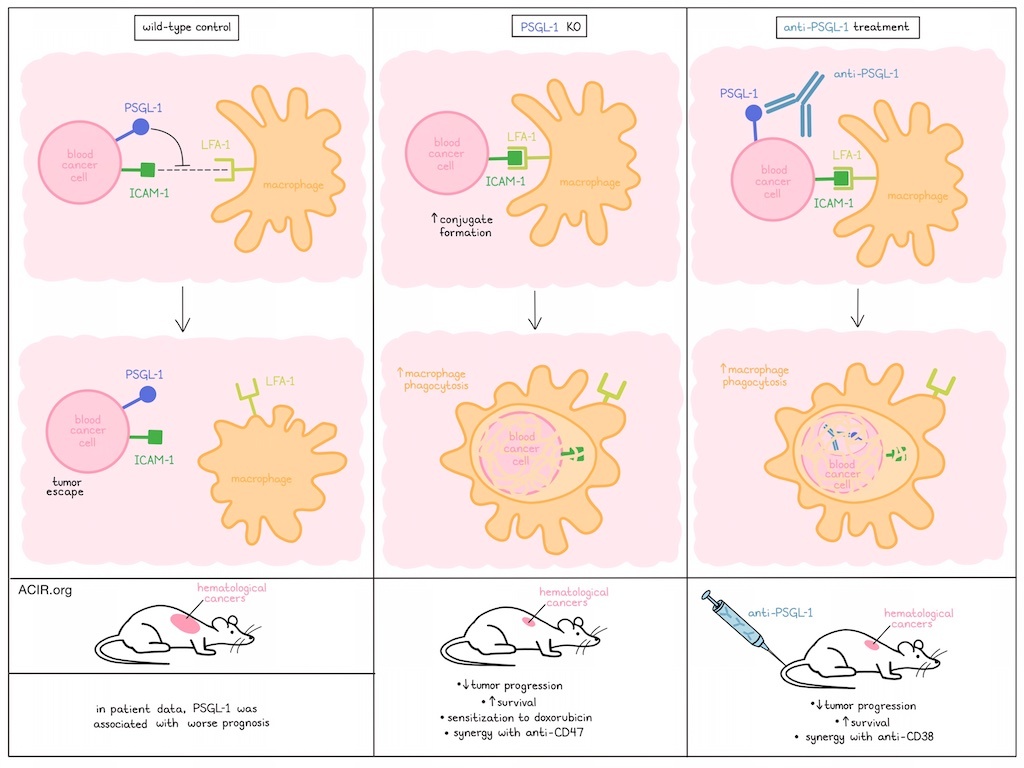
Tumor cells can evade immune detection through a wide range of mechanisms, not all of which are fully understood. In recent work by Zhong and Wang et al., P-selectin glycoprotein ligand 1 (PSGL-1) – a known molecule involved in adhesion, leukocyte trafficking, extracellular vesical generation, and T cell exhaustion – was identified as a novel immune checkpoint molecule that was expressed by a variety of blood cancers cells and hindered phagocytosis by macrophages. The results of investigations into PSGL-1 in this context, and into the possibility of targeting it for immunotherapy, were recently published in Science Immunology.
Initially, Zhong and Wang et al. analyzed the expression of immune inhibitory molecules in bulk RNAseq data of murine EL4 T cell lymphoma, which is resistant to numerous checkpoint inhibitors. This identified strong expression of CD43, CD44, CD155, Tim3, and PSGL-1 on EL4, and genetic deletion studies revealed that genetic ablation of PSGL-1 resulted in slower progression of EL4 tumors, with mice surviving longer, and more than 20% showing complete regressions. Similar results were seen in a syngeneic T-ALL model and in a subcutaneous myeloma model. Further, while EL4 tumors were largely resistant to treatment with doxorubicin, PSGL-1 KO EL4 tumors treated with doxorubicin showed 40% complete regressions, suggesting sensitization to chemotherapy.
To better understand how PSGL-1 impacts tumor development, the researchers evaluated wild-type and PSGL-1-KO EL4 tumors isolated from tumor-bearing mice. RNAseq, single-sample gene set enrichment analysis, flow cytometry, and IHC together showed that PSGL-1 deficiency was associated with increases in CD45+ cells, eosinophils, macrophages (including TAMs), DCs, and monocytic MDSCs. Depletion studies showed that CD4+ T cells, CD8+ T cells, and DCs were all dispensable for the antitumor effects associated with PSGL-1 deficiency. However, TAMs were required, as depletion of macrophages with anti-CSF1R restored tumor growth and abrogated the survival benefit associated with PSGL-1-deficiency. Knockout of CCL2, which is responsible for TAM recruitment, had a similar effect to TAM depletion.
Next, the researchers used bulk RNAseq data to interrogate markers of macrophage states and phenotypes. Wild-type and PSGL-1-deficient tumors showed similar expression of CD86, CD80, MHC-II, PD-L1, CD206, and genes associated with pro- and anti-inflammatory markers and cytokines, suggesting that macrophage states and phenotypes were generally unaffected by PSGL-1 deficiency. However, PSGL-1-deficient tumors were enriched for genes involved in innate immune response activation, the phagosome, and antigen presentation, suggesting that PSGL-1 may play a role in phagocytosis. To investigate this, the researchers used L1210 cells (murine B cell leukemia cells that are sensitive to macrophage phagocytosis) and found that PSGL-1-deficient L1210 cells were more readily phagocytosed by BMDMs in vitro than control L1210 cells.
Based on previous evidence that PSGL-1 can act as an adhesion molecule, the researchers investigated conjugate formation between PSGL-1 KO L1210 cells and BMDMs, and found that it was markedly increased with PSGL-1 KO L1210 cells compared to with wild-type L1210 cells, suggesting that PSGL-1 negatively regulates conjugate formation in this setting. The addition of anti-CD47 further increased phagocytosis of PSGL-1 KO cells by BMDMs, suggesting a potential rational combination.
When mixtures of WT and PSGL-1 KO tumor cells (L1210 or EL4) were injected intraperitoneally into WT mice, PSGL-1 KO cells were eliminated more efficiently. Across a variety of other tumor models, including different tumor cell lines (L1210, EL4), delivery methods (intraperitoneal, subcutaneous, intravenous), and recipient mice (wild-type, RAG-1 KO mice lacking T cells and B cells), PSGL-1-deficient tumors consistently showed reduced tumor progression and longer mouse survival compared to wild-type controls. As PSGL-1 is also expressed in macrophages and T cells, the researchers evaluated PSGL-1-deficient mice, but found that this did not impact the growth of WT or PSGL-1-deficient tumors.
Despite previous evidence that PSGL-1 regulates cell-to-cell adhesion, signal transduction, and immune suppression by interacting with receptors including P-selectin, SiglecE, and VISTA, knockout studies revealed that none of the receptors were involved in the increased phagocytosis mediated by PSGL-1. However, focal adhesion kinase (FAK), Ca2+, PI3K, integrin signaling (Syk and Src kinases), and cytoskeletal reorganization were all required for effective phagocytosis. Looking more closely at integrin receptors, the researchers found that interactions between LFA-1 (CD11a–CD18 heterodimer) on BMDMs and ICAM-1 on tumor cells were required for the effective phagocytosis of PSGL-1 KO tumor cells. However, as levels of LFA-1 and ICAM-1 were consistent between wild-type and PSGL-1 KO tumor settings, the researchers hypothesized that PSGL-1 likely disrupts physical interactions between ICAM-1 and LFA-1 during macrophage phagocytosis. Demonstrating this, inhibition of ICAM-1/LFA-1 interactions had no impact on phagocytosis in WT tumors, but abrogated the increased phagocytosis observed in PSGL-1-deficient settings.
To investigate the potential relevance of PSGL-1 in human cancers, the researchers analyzed RNAseq datasets from TCGA and another public database, and found that high expression of SELPLG (encoding human PSGL-1) was associated with poor patient survival in AML and MM. Further, hPSGL-1 was expressed at high levels in freshly isolated tumor cells from patients with T-ALL, AML, and MM, and at low levels in B-ALL.
Exploring the potential for therapeutic targeting of hPSGL-1, the researchers generated a humanized monoclonal antibody that specifically recognized both soluble and membrane-bound hPSGL-1. Toxicological studies on four cynomolgus macaques showed that the antibody was well tolerated, and in vitro studies showed that it effectively triggered mouse BMDMs to phagocytose various human hPSGL-1+ blood cancer cell lines and freshly collected patient samples. In NOD/SCID mice-bearing human xenograft tumors, anti-hPSGL-1 suppressed growth of T-ALL, MM, and AML tumor cell lines. The antitumor efficacy of anti-hPSGL-1 was comparable to that of anti-CD38 (darzalex), and when used in combination, the antibodies more strongly reduced tumor growth and prolonged survival compared to either alone.
Overall, these results suggest that PSGL-1 expression on hematological tumors protects cancer cells from macrophage phagocytosis by inhibiting interactions between ICAM-1 on tumor cells and LFA-1 on macrophages. In mice, knockout or antibody blockade of PSGL-1 reduced the progression of various hematological tumors, sensitized tumors to doxorubicin chemotherapy, and showed synergy in combination with anti-CD47 or anti-CD38 antibodies. In combination with patient data in which hPSGL-1 correlated with worse survival, these results suggest that PSGL-1 can act as an immune checkpoint and could serve as a novel target for immunotherapy.
Write-up and image by Lauren Hitchings




