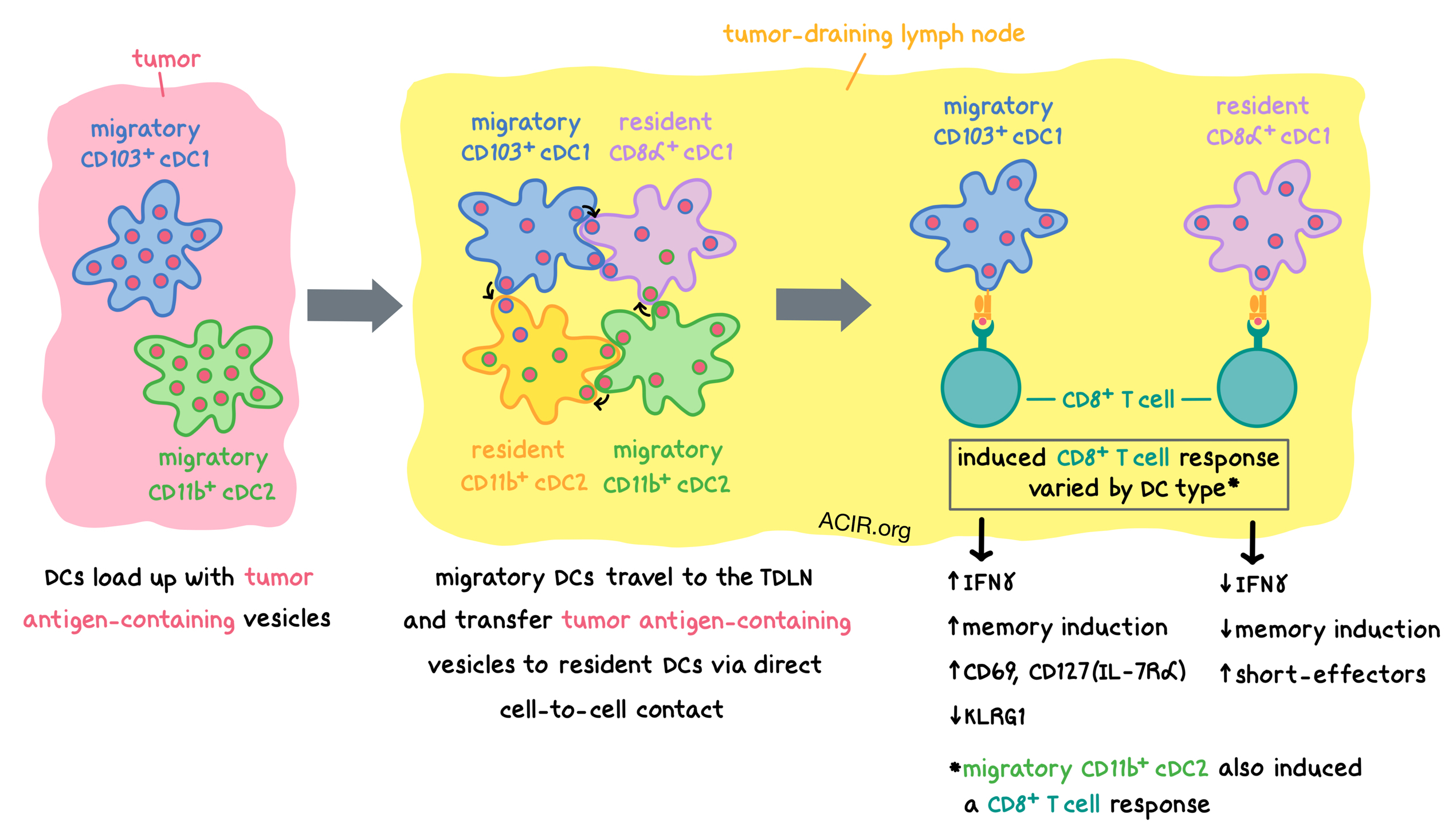
It is well known that the initiation of an antitumor T cell response begins with priming by dendritic cells (DCs) inside the tumor-draining lymph node (TDLN). But how do migratory DCs, which pick up antigens at the tumor site, transfer the tumor antigens to LN-resident DCs and enable T cell priming? To answer this question, Ruhland and Roberts et al. utilized various direct imaging techniques and found that DCs employ a vesicle-based “messenger” system to spread tumor antigens across DC subsets within TDLNs. The results were recently published in Cancer Cell.
The researchers began by modifying B16F10 melanoma cells to express the ZsGreen fluorophore, which localizes in intracellular compartments, and grew the B16ZsGreen tumors in mice that ubiquitously expressed membrane-bound tdTomato (mT+ mice). Confocal microscopy and flow cytometry showed that within the tumor microenvironment, nearly 100% of host myeloid cells (specifically tumor-associated macrophages, monocytes, CD103+ cDC1s, and CD11b+ cDC2s) contained ZsGreen-labeled vesicles. Furthermore, over 75% of ZsGreen-containing vesicles stained positive for melanoma-associated antigens (gp100 and tyrosinase).
Within the TDLNs, high-resolution lattice light sheet (LLS) microscopy demonstrated that, once again, ZsGreen was encapsulated within vesicles inside host cells. Approximately 40% of each of the four analyzed DC subsets in TDLNs (migratory CD103+ cDC1s, resident CD8α+ cDC1s, migratory CD11b+ cDC2s, and resident CD11b+ cDC2s) stained positive for ZsGreen, and the majority of the ZsGreen+ DCs, including resident DCs, also contained melanoma-associated antigens.
Inside the cells within the lymph nodes, ZsGreen-containing vesicles were found both near the cell membrane and deeper within the cytoplasm. CCR7 was required for cells to stain positive for ZsGreen within TDLNs, consistent with the conclusion of previous studies which demonstrated CCR7 as a requirement for DC migration from the tumor to the TDLN. The number of ZsGreen-containing vesicles within migratory DCs in the TDLNs was less than half, and the fluorescence intensity reduced 10-fold, compared with migratory DCs in the tumor. Within TDLNs, there were more resident DCs than migratory DCs, and the two populations exhibited comparable fluorescence brightness. These results suggested that ZsGreen was actively redistributed via vesicles among various DC subsets within the TDLN.
Coculture of ZsGreen+ mT+ DCs (obtained from the lymph nodes of tumor-bearing mice) with unlabeled recipient DCs confirmed that vesicles comprising ZsGreen enveloped by mT+ membrane were transferred from ZsGreen+ DCs to recipient DCs. Further in vitro experiments showed that both migratory DC subsets (CD103+ cDC1s and CD11b+ cDC2s) efficiently transferred vesicles to recipient DCs, and that resident cDC1s (particularly CD8α+ cDC1s) were the best recipients, accumulating the most ZsGreen+ vesicles. In vivo, both resident DC subsets (CD8α+ cDC1s and CD11b+ cDC2s) accumulated significant amounts of ZsGreen+ vesicles.
Exosome release, DC apoptosis, and LFA-1 did not play significant roles in antigen transfer between DCs. MHC-I cross-dressing (the transfer of peptide-MHC-I complexes from donor cells to recipient cells) was observed between all DC subtypes in the context of ZsGreen transfer, but at low levels compared with vesicle transfer. Thus, the researchers suspected that tumor antigen-containing vesicle transfer occurred via direct cell-to-cell contact. To explore this hypothesis, Ruhland and Roberts et al. came up with a real-time, two-photon imaging strategy and, together with high-resolution, real-time LLS microscopy, used it to analyze excised TDLNs from mice bearing transgenes that allowed for distinct fluorescent labeling of cDC1s, cDC2s, resident cDC2s, and monocytes. Together, the imaging experiments demonstrated that:
- various types of DCs came in direct contact with each other;
- the contact was persistent, lasting up to ten minutes or more; and
- membrane material was exchanged between the cells in contact.
The direct cell-to-cell transfer of vesicles was confirmed in a co-culture experiment using a conventional confocal microscope with a wider field of view. An individual dendritic cell could sequentially transfer vesicles to multiple recipient cells and remain viable. Antigen uptake was always observed to be a result of direct cell-to-cell contact, and was not due to cell-free or apoptotic debris uptake. Such antigen transfer was also observed in vivo within the TDLNs of B16ZsGreen tumor-bearing mice.
Next, using an inducible ZsGreen system, the researchers showed that various myeloid populations were filled with antigen sequentially, rather than all at once, and this was consistent with direct cell-to-cell antigen transfer. Within the tumors, neutrophils, macrophages, and monocytes were maximally ZsGreen+ at approximately the same time as the tumor cells themselves, while DCs took longer to fully load with antigen. Migratory DCs loaded with ZsGreen appeared in TDLNs as early as 3 days after ZsGreen induction, followed by transfer of ZsGreen+ vesicles to resident DCs by day 5; loaded monocytes appeared last, by day 7. The amount of antigen within DCs grew with tumor size, as migratory DCs took up higher quantities of tumor antigen and transferred more antigen to resident DCs within TDLNs.
To confirm the relationship between vesicle transfer and antigen presentation to T cells, Ruhland and Roberts et al. generated B16F10 tumor cells that expressed ZsGreen fused to the OT-I and OT-II OVA peptides. CD4+ OT-II T cells were induced to proliferate only by the ZsGreen+ migratory CD11b+ DCs, while CD8+ OT-I T cells were induced by ZsGreen+ migratory CD103+ DCs, migratory CD11b+ DCs, and resident CD8α+ DCs, indicating a cross-presenting capability for the latter two subsets when they contain a ZsGreen+ vesicle. Gene expression analysis of OT-I T cells showed that, compared to migratory CD103+ and CD11b+ DCs, CD8α+ DCs induced a CD8+ T cell response that featured decreased IFNγ pathway and memory induction, and increased gene modules associated with short-term effectors, which are less effective at tumor rejection than memory phenotypes. Compared to T cells stimulated by CD8α+ DCs, T cells stimulated by CD103+ DCs expressed increased CD69 and CD127 (IL-7Rα), and decreased KLRG1.
Exploring the vesicle-based antigen transfer in a setting other than tumors, the researchers utilized mice that ubiquitously express ZsGreen in the skin. Under steady state conditions, various myeloid populations within the skin were equally loaded with ZsGreen. However, in the draining lymph node (DLN), migratory CD103+ DCs had a lot more antigen than other DC subtypes. Increased transfer of antigen to resident DCs in the DLN was observed when irradiation was used to induce inflammation. Thus, the transfer of antigen from migratory DCs to resident DCs within draining lymph nodes was dependent on the context.
Overall, Ruhland and Roberts et al. demonstrated that DCs take up antigens within the tumor and carry them within vesicles to TDLNs, where they transfer the antigen-loaded vesicles to other DC subtypes, including LN-resident DCs, via direct cell-to-cell contact. Vesicle-containing DCs were able to activate T cells, and different types of DCs induced different T cell responses. These results could help predict immune responses and inform the design of future immunotherapies.
by Anna Scherer




