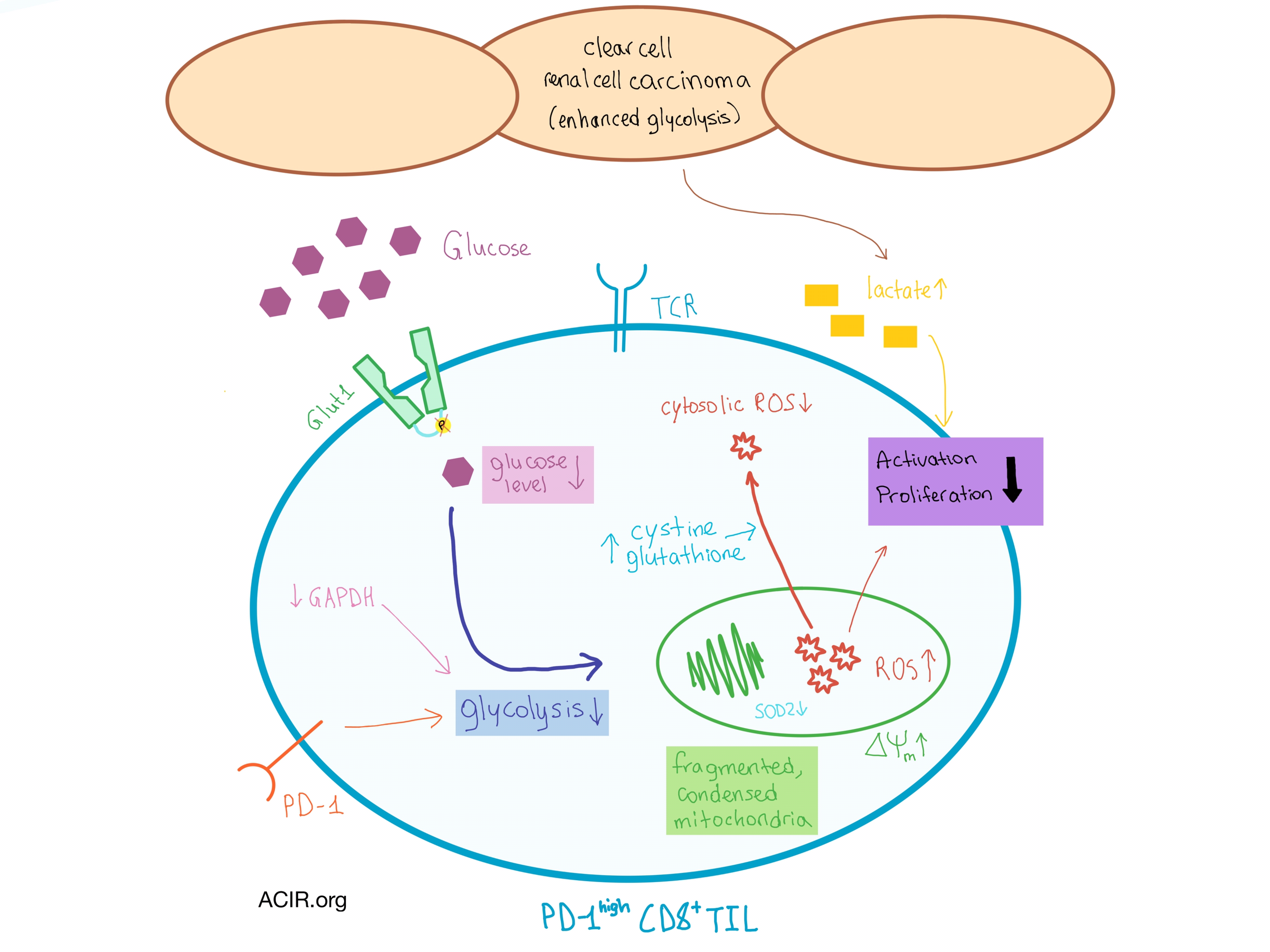
Despite extensive CD8+ T cell infiltration, clear cell renal cell carcinoma (ccRCC) responds only moderately to checkpoint blockade therapy, suggesting other immunosuppressive mechanisms. One mechanism by which tumors can avoid effector T cell function is by metabolically inhibiting the tumor-infiltrating lymphocytes (TIL). Siska et al. used a combination of mass and flow cytometry, microscopy, and metabolic perturbations to examine the metabolic pathways and mitochondrial state in TIL from 54 patients with ccRCC and revealed how much energy and the environment matter.
The ccRCC CD8+ TIL exhibited the phenotype of an effector memory T cell and uniformly expressed high levels of PD-1, indicative of their suppressed or exhausted state. Isolated CD8+ TIL were limited in activation and proliferation compared to CD8+ T cells from the peripheral blood. Probing the metabolic machinery revealed that despite normal expression of hexokinase and the glucose transporter Glut1, the glucose uptake process in CD8+ TIL was defective and comparable to resting CD8+ T cells. Further analysis showed that Glut1 in this cell population was not phosphorylated, possibly indicating that the glucose transporter was unable to import extracellular glucose into the cell, and that downstream GAPDH levels, key to efficient glycolysis, were reduced. Interestingly, ccRCC CD4+ TIL were only slightly affected, suggesting a differential response to the environment by the two T cell types.
Stimulating the CD8+ TIL did not result in increased glucose uptake, nor did exposure to low glucose result in further suppression, indicating a metabolic adaptation that is not dependent on exogenous glucose. It became clear that this adaptation was not due to low exogenous glucose levels, as the levels of interstitial glucose in the tumor tissue were similar to adjacent normal tissue. Such observation was surprising, as tumor cell metabolism in general, and the von Hippel-Lindau (VHL) tumor suppressor gene mutations characteristic of ccRCC in particular, often lead to increased glycolysis in cancer cells. However, the lactate levels in ccRCC interstitial fluid were elevated, pointing toward the role of other metabolic pathways, including PD-1 signaling.
In addition to the metabolic impairments, changes were also observed in the mitochondria, which play a key role in energy production and generation of reactive oxygen species (ROS). Although the mitochondrial mass and expression of electron transport components and transcription factors were normal, the ccRCC CD8+ TIL had small, dispersed mitochondria that were highly fragmented and in a condensed configuration, with an elevated mitochondrial membrane potential. High levels of ROS were found inside the mitochondria, likely due to the hyperpolarization as well as the decreased expression of SOD2, which helps neutralize mitochondrial ROS. However, the total cellular ROS was not elevated, as the expression of SOD1 (which plays a role in neutralizing cytoplasmic ROS) remained normal. In addition, CD8+ TIL utilized an adaptive cytosolic mechanism (cystine uptake and subsequent increased glutathione production) to neutralize ROS. The upregulation of cytosolic ROS neutralization may have contributed to defective T cell activation, as cytosolic ROS is important to calcium signaling in activated T cells.
As the CD8+ TIL adapted to decreased dependence on glucose and high mitochondrial ROS, the metabolic defects became persistent, even in the presence of additional nutrients and treatments, which only partially increased the activation of impaired TIL. Thus, metabolic adaptations within the tumor microenvironment appear to be imprinted upon the existing infiltrating T cells and have limited the activation and effectiveness of CD8+ TIL in ccRCC. Finally, CD8+ T cells demonstrating high membrane polarization can be found in the peripheral blood of ccRCC patients, potentially identifying tumor-experienced T cells.
by Anna Scherer




