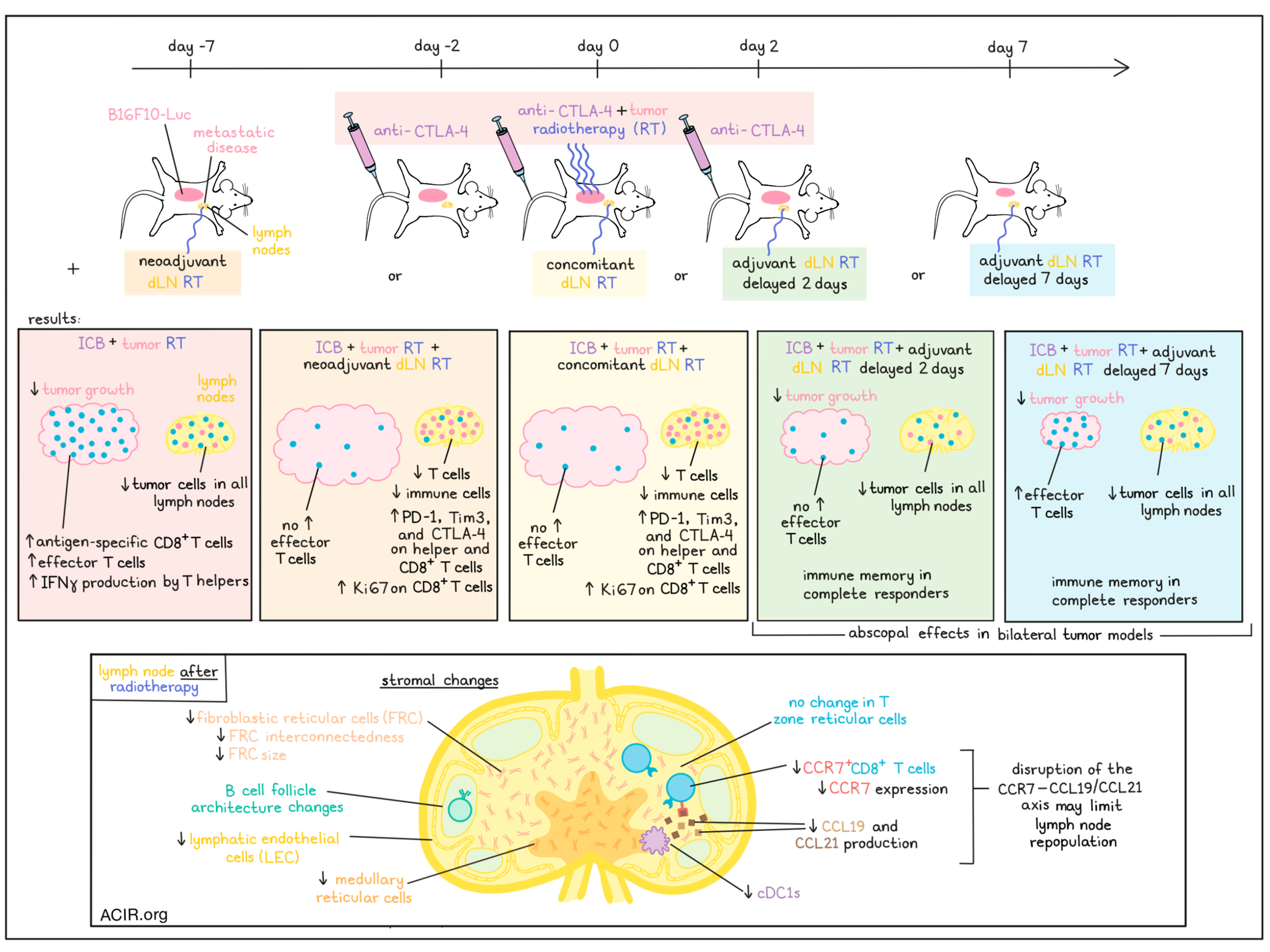
Even though the combination of radiotherapy and immune checkpoint blockade (ICB) has great potential to work synergistically, clinical efficacy has been limited. Irradiation of the draining lymph nodes (dLNs) to target metastases might be to blame for this, as these are essential places for antigen presentation and priming of T cells, needed for ICB efficacy. Telarovic et al. investigated the timing of radiotherapy (RT) of the dLN in combination with tumor RT and ICB in a murine model for metastatic disease in a recent Nature Communications publication.
For a murine model tumor with nodal involvement, luciferase-expressing B16F10 cells were developed to allow for analysis of tumor cells in the dLNs. On day 6 after injection, 80% of mice had tumor cells in the dLNs. A small-animal image-guided radiotherapy platform was used to specifically irradiate or spare LNs. First, to mimic the current clinical situation, dLNs were irradiated at the same time as the tumor. RT of just the tumor and RT of both tumor and dLN resulted in tumor growth inhibition, but no significant differences were observed between these two irradiated groups.
To assess the effects of these RT strategies on the efficacy of ICB, mice were treated with anti-CTLA-4 on days -2, 0, and 2, with respect to RT (day 0) of either the tumor alone or tumor plus dLNs. Tumor responses were monitored for 60 days, and the tumor immune microenvironment was studied within 10 days after RT. Tumor-only RT plus ICB was much more successful than RT or ICB alone in controlling tumor growth. However, mice treated with tumor/dLN RT plus ICB had a short delay of tumor growth, but then progressed. In the immune compartment, the tumor-only RT plus ICB group had a significant increase in CD8+ T cells as compared to controls or the tumor/dLN RT plus ICB group. Most of these CD8+ T cells expressed PD-1, Tim3, and CD39, suggestive of an antigen-specific tumor-reactive phenotype.
The researchers hypothesized that delaying the dLN RT (adjuvant) might be more effective to allow for immune priming in the dLN in response to ICB, while maintaining the benefit of dLN RT. Based on the observed immune influx data, the adjuvant treatment was tested at 2 days or 7 days after tumor irradiation, with ICB treatment at days -2, 0, and 2. Day 7 dLN RT maintained the tumor growth inhibition benefit of combination treatment, and even day 2 was superior to day 0 dLN RT. At day 7, before the day 7 dLN RT, the tumor-only RT plus ICB and the tumor/day 7 dLN RT plus ICB groups had increases in the number of effector T cells in the tumor, but day 2 RT dLN plus ICB did not. The researchers also assessed dLN RT before tumor RT (neoadjuvant), by providing dLN RT on day -7, tumor RT on day 0, and ICB on days -2, 0, and 2. However, this neoadjuvant schedule had similar effects as concomitant tumor and dLN RT.
Of all treatment modalities discussed so far, only the tumor-only RT plus ICB and the adjuvant dLN and tumor RT plus ICB reduced luciferase-expressing tumor cells in all dLNs. To determine whether immune memory responses were induced, complete responders were rechallenged with B16F10-Luc tumor cells. Most of the mice treated with tumor-only and delayed dLN RT rejected the B16F10 tumors.
To determine whether delayed dLN RT affected distant disease control, two bilateral tumor models (WT B16F10 and MC38) were assessed, with a clinically relevant RT schedule plus combined anti-CTLA-4 and anti-PD-1 ICB. Since WT B16F10 is less immunogenic than its luciferase counterpart, this model is immune cold. Delayed dLN RT resulted in better treatment responses than concomitant tumor/dLN RT, largely due to tumor growth inhibition of the distant, non-irradiated tumor.
To determine what processes in the LN induced by RT were responsible for the effects observed, the immune cell composition of the dLN was determined. Significant immune cell depletion was observed in the irradiated dLN in the neoadjuvant setting, and all major T cell subsets decreased after RT. In dLNs irradiated at the same time as the tumor, similar immune cell depletion was observed. In mice treated with tumor RT only, IFNγ expression increased in T helper cell compartments in the dLN on day 4 after RT, while in mice treated with concomitant or neoadjuvant dLN RT, higher expression of PD-1, TIM-3, and CTLA-4 was observed in CD8+ and helper T cell subsets in the dLN. Additionally, the proliferation marker Ki67 was upregulated in CD8+ T cells of dLNs that received neoadjuvant and concomitant RT.
Since stromal cells in the LNs are important for compartmentalization of antigens, APCs, and lymphocytes, the researchers investigated how RT impacted the composition of fibroblastic reticular cells (FRCs), lymphatic endothelial cells (LECs), and blood endothelial cells (BECs). LNs of healthy mice received a single high dose RT, which resulted in changes in the architecture of B cell follicles, with a less interconnected meshwork of FRCs, which also had a smaller cell surface. RT induced a decrease in LECs and FRCs, as well as in medullary reticular cells, while the T zone reticular cells remained similar.
Assessing chemokines produced in stromal cells of LNs showed a reduction in CCL19 and CCL21 in the LNs after RT. CCR7 is the receptor for these chemokines and is an important chemotactic receptor for LN homing of both T cells and dendritic cells. Expression of CCR7 on T cells, and the proportion of CCR7+CD8+ T cells were reduced in the LNs after RT. The researchers hypothesized that the disruption in the CCR7–CCL19/CCL21 axis might be the cause of the limited repopulation of RT-treated dLNs, as there was also a reduction in conventional type I DCs (cDC1s) in response to dLN RT.
The data presented here suggest that radiation of the dLN affects the efficacy of combined radio- and immunotherapy, as it leads to disturbance of CCR7–CCL19/21 signaling, limiting cross-presentation by cDC1s and T cell priming. Therefore, adjuvant (delayed) dLN RT might improve treatment responses, but the optimal timing in patients will have to be determined.
Write-up by Maartje Wouters, image by Lauren Hitchings




