Although immune checkpoint blockade (ICB) therapy has shown impressive benefits in treating lung cancer, only a minority (~20%) of patients with non-small-cell lung cancer (NSCLC) respond to anti-PD-1/anti-PD-L1 therapy. The reasons are incompletely understood, but the tumor microenvironment and its inflammatory state could play a key role. Recently reported in Science Translational Medicine, Breitenecker, Homolya and Luca et al. investigated the role of A20, a potent anti-inflammatory TNFα-induced protein, in lung adenocarcinoma (LUAD) progression and ICB response.
To begin, the authors probed the TCGA database for evidence of A20 gene alterations in lung cancer. Roughly half of LUAD patients had deletions in A20 (predominantly ‘shallow’, meaning potentially heterozygous), and A20 mRNA was downregulated in tumors relative to healthy lung tissue. In KRAS-mutant LUAD, the majority of patients had A20 deletions. Importantly, low A20 expression correlated with a worse prognosis.
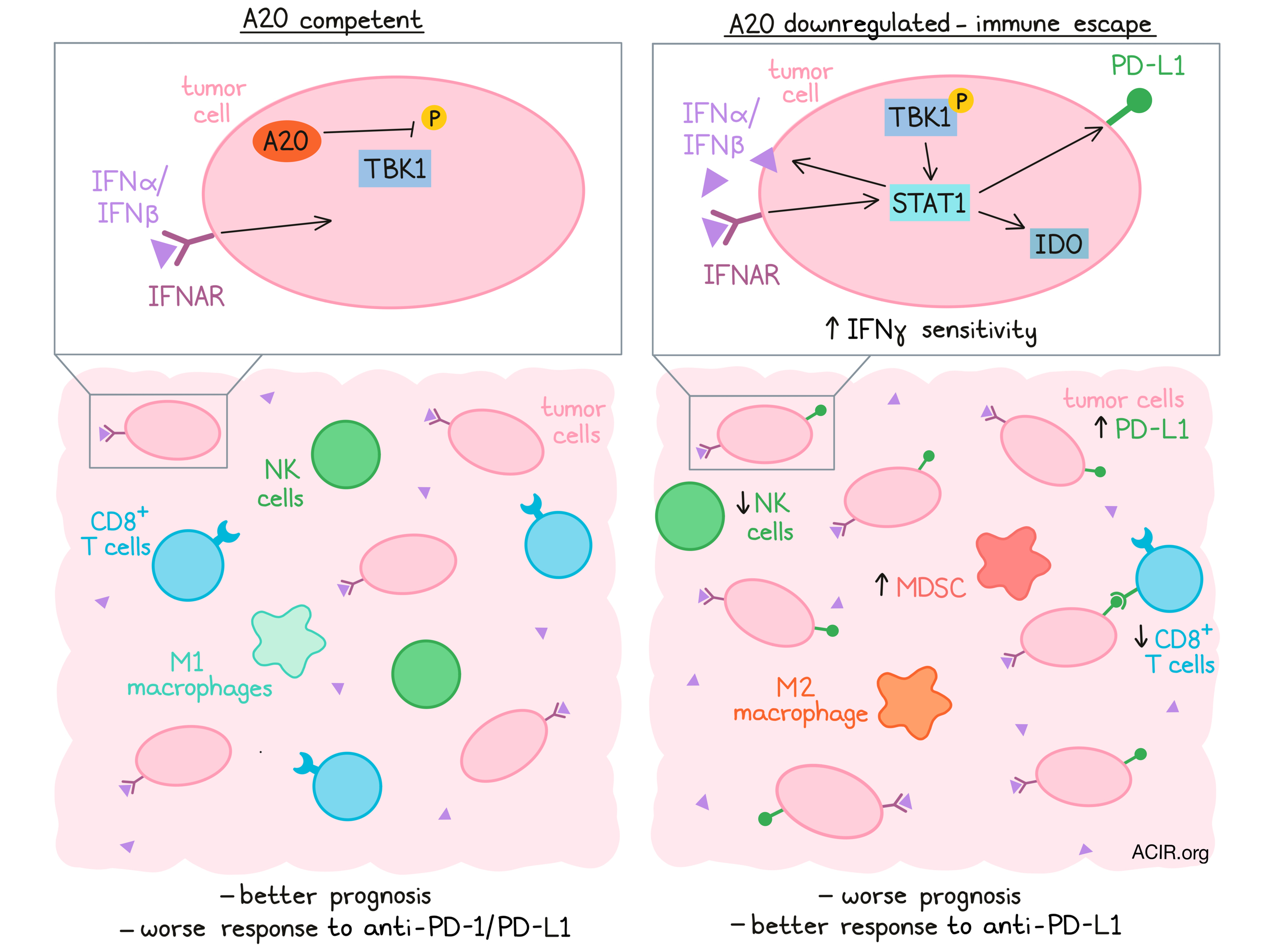
Investigating these findings in an experimental LUAD model, the researchers generated KRASG12D x A20fl/fl (KA) mice. By administering a Cre-expressing adenovirus intranasally, A20 could be deleted in lung epithelial cells, resulting in the formation of A20-deficient LUAD. Compared to mice with KRAS mutation alone (K mice), KA mice grew larger, more advanced tumors, and had reduced survival. Similarly, mice with KRAS/p53-driven LUAD (KP mice) had worse survival when A20 was deleted (KPA model). Interestingly, A20 mRNA was reduced in K mouse tumors (despite their supposed A20 competence) relative to healthy lung tissues, although not as much as in KA tumors. Altogether, these findings in both patient data and mouse models suggest that A20 curbs LUAD growth, and its downregulation can promote tumor progression.
Breitenecker, Homolya, Luca, and the team next considered how A20 might restrict tumor development. A20 deletion did not affect in vitro LUAD proliferation or growth in immunodeficient (NSG) mice, suggesting that A20 did not act in a tumor cell-intrinsic manner. In KP tumors, A20 loss reduced markers of proliferation (Ki67), but also markers of apoptosis (cleaved caspase 3), again likely not responsible for the striking changes in tumor progression. The authors then turned their attention to the tumor microenvironment. Notably, A20-deficient KPA tumors had fewer CD8+ T cells and NK cells and more myeloid-derived suppressor cells. Likewise, in a LUAD patient cohort, A20 expression correlated positively with gene expression for CD8+ T cells, NK cells, M1 macrophages, perforin-1, and granzyme A, and negatively with M2 macrophages and Tregs. Broadly, these results demonstrated that A20 loss coincided with a suppressive tumor microenvironment conducive to tumor growth.
Next, the researchers more specifically probed the tumor T cell compartment. In patient LUAD samples, immunohistochemistry identified a positive correlation between A20 expression and CD8+ T cell abundance and PD-L1 expression in tumor cells. Furthermore, in a TCGA dataset of patients with KRAS-mutant LUAD, a gene signature for CD8+ T cell infiltration was higher in patients with wild-type A20 than with A20 deletion. Studying T cells in their mouse models, the team observed that compared to KP tumors, KPA tumors had reduced CD8+ T cell infiltration and cytolytic marker expression, and when transplanted into wild-type C57 mice, resulted in significantly reduced survival. In fact, antibody-mediated depletion of CD8+ T cells in the KP model (either transplanted or spontaneous) reduced mouse survival to match that of the KPA model. Taken together, these results indicated that CD8+ T cell infiltration and control of LUAD tumors depends on A20.
The authors next investigated which signaling pathways could mediate the effects of A20 loss in LUAD tumors. Relative to KP tumors, KPA tumors were enriched in IFNγ- and IFNα-responsive gene sets, as well as STAT1, a downstream mediator of IFN signaling. In line with this finding, IFNγ stimulation of KPA cells increased expression of PD-L1 and IDO (also downstream of STAT1) more so than KP cells. However, the link to A20 was still unclear. Previously, the research group had found that A20 regulates the activity of the kinase TBK1, involved in IFNα/β-receptor (IFNAR) and STAT1 signaling. This pathway was relevant in LUAD as well; KPA cells had greater TBK1 phosphorylation than KP cells, and inhibiting TBK1 reduced downstream STAT1 and PD-L1 expression. Showcasing the importance of IFN signaling in vivo, transplanted KPA had a greater intratumoral CD8+ T cell abundance when IFNAR was deleted. Furthermore, in a spontaneous LUAD model, KA x IFNARfl/fl mice (deficient in lung IFNAR signaling) demonstrated improved survival, reduced tumor burden, and increased T cell abundance compared to their IFNAR-competent counterparts. These findings highlight the role of IFN signaling in CD8+ T cell exclusion induced with A20 downregulation.
To conclude, the team considered the therapeutic implications of their results in the context of ICB therapy. Previously, IFN signaling has been associated with improved ICB response, a positive indication given the above findings. And in fact, an A20 loss-of-function gene signature correlated positively with anti-PD-1 response (overall and progression-free survival) in a melanoma patient cohort. Testing this hypothesis in mouse models, the researchers generated PD-L1-knockout KP and KPA lines. Compared to KPA, mice transplanted with PD-L1 KO KPA cells had improved survival and CD8+ T cell tumor infiltration – now on par with KP tumors, for which PD-L1 deletion had little effect. Moreover, while KP mice were unresponsive to anti-PD-L1 therapy, KPA tumors treated with anti-PD-L1 showed reduced tumor burden and increased T cell infiltration.
In this exciting work, Breitenecker, Homolya, and Luca et al. uncovered a pathway underlying immune escape in LUAD, but also conferring a therapeutic opportunity. A20 downregulation, by altering TBK1/STAT1 and IFN signaling, molded an inhospitable tumor microenvironment, but at the same time, rendered these tumors more susceptible to anti-PD-L1 therapy. These findings support the advantageous application of immunotherapies and highlight the importance of the tumor microenvironment in cancer treatment.
Write-up by Alex Najibi, image by Lauren Hitchings




