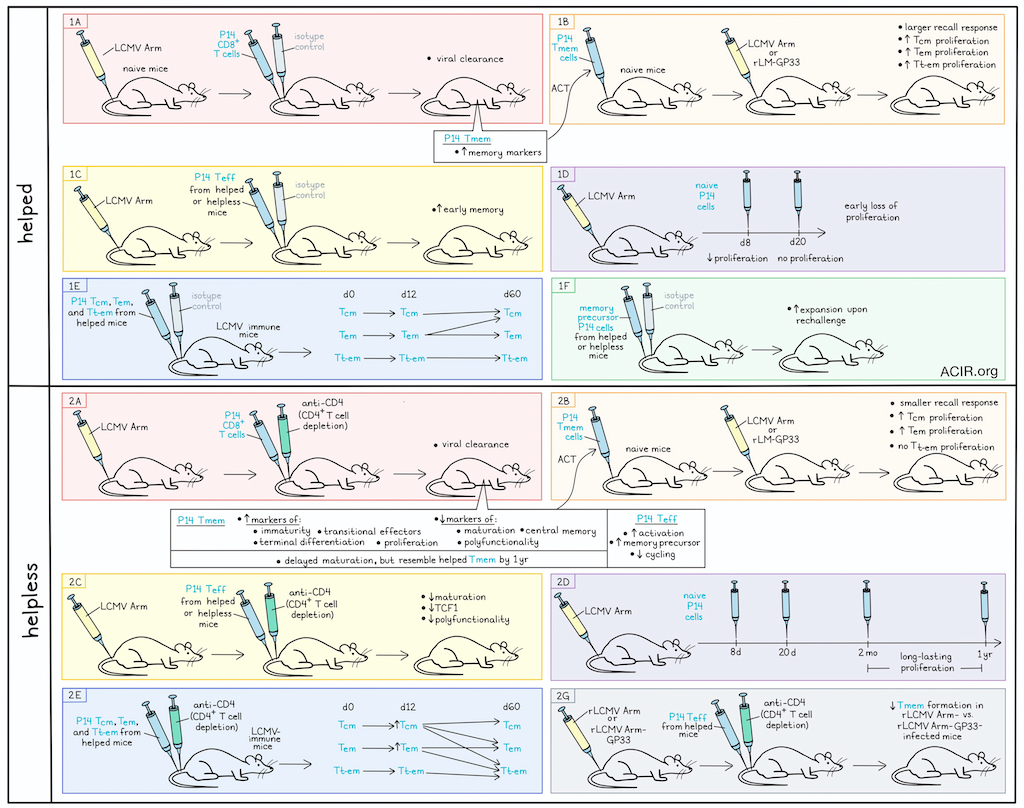
During acute infections, the primary immune response depends on CD4+ T cells providing help to CD8+ T cells. However, many effector CD8+ T cells (Teff) can respond to pathogens independently of helper responses, but these cells respond poorly to pathogen rechallenge. Van der Heide et al. investigated this helpless CD8+ T memory (Tmem) recall in acute pathogen models, and their results were recently published in Immunity.
The researchers developed a model with the acute LCMV Armstrong (Arm) strain, transferring naive LCMV-specific P14 CD8+ T cells into isotype-treated (helped) or transiently CD4+ T cell-depleted (helpless) C57BL/6 mice before infection. Both conditions cleared the virus and generated P14 Tmem pools. When helped or helpless P14 Tmem were transferred into naive mice and challenged with high-dose recombinant Listeria monocytogenes (LM) expressing the LCMV GP33-41 determinant (rLM-GP33) or with the original LCMV Arm strain, the helpless Tmem cells had smaller recall responses.
Helpless P14 Tmem had more immature phenotypes with increased expression of terminal differentiation markers, while the helped cells expressed more memory-related markers. Two distinct clusters were observed, with the helpless cells having more transitional effector-like and terminally differentiated phenotypes, with fewer central memory (Tcm) phenotypes. Further, the helpless cells had elevated Ki67 and granzyme A expression, indicative of proliferation, increased T-bet and reduced TCF1 and FOXO1 expression, indicative of impaired maturation, and limited polyfunctionality. This phenotype suggested these cells remained at an earlier memory stage.
The researchers hypothesized that maturation of helpless cells occurs at a delayed pace, and investigated the long-term fates of these helpless CD8+ Tmem cells. The helpless cells remained at similar frequencies, and had similar, but delayed maturation trajectories to helped cells, resulting in a helped-like Tmem phenotype at >1 year after LCMV infection.
To determine the maturation potential of the helpless cells, cell populations were sorted and traced after transfer into naive mice. After 90 days, helped and helpless Tcm and Tem subsets had undergone proliferation and were found at similar numbers in the spleen. Transferred terminally differentiated Tem (Tt-em) cells, however, had minimal proliferation and a 10-fold reduction in the spleen. The maturation trajectories between helpless and helped Tmem subsets were similar, and maintained a Tcm phenotype, while most of the Tem cells converted to a Tcm phenotype. About half of the remaining Tt-em cells acquired Tem or Tcm markers. Therefore, maturation and survival capacities of these subsets were largely preserved across helped and helpless populations. Further experiments showed that response to rechallenge was indistinguishable at the later stage, suggesting the helpless T cell memory deficit is temporary.
Next, the researchers conducted scRNAseq of helped and helpless Teff cells. Clustering of the data revealed that helpless cells had larger activated and smaller cycling clusters and increased amounts of memory precursor-cluster cells. Overall, the helpless Teff cells had slightly reduced activation, but no major differences between helped and helpless were found in functional profiles.
Given the similarity between helped and helpless cells at the peak of infection, the researchers hypothesized that the distinctive helpless Tmem defects might be acquired in the post-effector stage. To assess this, helped and helpless Teff cells were transferred into helped and helpless hosts 8 days after LCMV Arm infection, and Tmem formation was monitored. At 49 days, both helped and helpless cells acquired early memory phenotypes in infection-matched helped hosts, but their phenotypic maturation was reduced in helpless recipients. In these helpless hosts, cells had reduced TCF1 expression and polyfunctionality, independent of the origin of the donor Teff cells.
To determine the memory potential of helped and helpless memory precursor cells, P14 cells expressing a reporter for intracellular TCF1 expression were generated. Helped or helpless memory precursor cells were transferred into helped infection-matched recipients to assess Tmem formation. As the Tmem pool was small, hosts were rechallenged with virus after 49 days, and this revealed similar secondary Teff expansion of both helped and helpless cells.
To investigate what drives the early memory-phase helpless cells to proliferate, naive P14 cells were labeled and transferred into helped and helpless P14 chimeras at various timepoints after LCMV infection, and retrieved from spleens 7 days later. In helped recipients, proliferation of transferred responder cells declined after day 8, and was almost absent 20 days post-infection. In helpless recipients, proliferation continued for 2 months to up to 1 year. The extent of the proliferation in helpless mice correlated with the phenotypic profiles of the originally generated Tmem cells, with increased immature Tmem phenotypes leading to more proliferation.
To determine the effect of prolonged antigen presentation on Tmem fates, labeled helped Tcm, Tem, and Tt-em cells were transferred into LCMV-immune helped and helpless hosts, and their proliferation and phenotypes were assessed 12-60 days later. Helpless host environments resulted in robust division of Tcm and Tem cells within 12 days, with minimal Tt-em proliferation. Within 60 days post-transfer, Tcm cells differentiated into Tcm, Tem, and Tt-em populations in helpless host spleens, while they remained of Tcm phenotype in helped hosts. About half of Tem cells converted into Tt-em in helpless hosts, while about half acquired the Tcm phenotype in helped hosts. Therefore, prolonged viral-antigen presentation in helpless mice may result in intermittent virus-specific Tmem cell engagement, resulting in differentiation with a delay in maturation.
To determine whether altered inflammatory characteristics under helpless conditions play a role in the delayed Tmem maturation, the researchers engineered a recombinant LCMV Arm strain (rLCMV Arm-ΔGP33) not recognized by the P14 cells. No proliferation of P14 cells was detected in helpless mice challenged with this virus, suggesting the inflammatory alterations alone were not sufficient to drive antigen-independent cell division (similar results were observed with OVA-specific OT-I cells). To assess Tmem formation in the absence of prolonged antigen presentation, helped congenic P14 Teff cells generated in rLCMV Arm-infected mice were transferred 8 days post-infection into helpless hosts that were 8 days post-infection with either rLCMV Arm or rLCMV Arm-ΔGP33. After 65 days, Tmem formation was reduced in the rLCMV Arm-infected hosts. However, Tmem formation was observed in the rLCMV Arm-GP33-infected helpless mice, which may be due to a lack of acquisition of helpless defects. The researchers confirmed their data by repeating experiments in three additional models of acutely resolving infection, although the magnitude of the distinctions and the kinetics varied by model).
The data in this study provide important clues to CD8+ T cell memory formation, and suggest that the memory defects of specific CD8+ T cells due to absent CD4+ T cell help after acute infection is a result of delayed maturation in response to ongoing antigen exposure. However, Tmem maturation does occur with reduction of antigen exposure over time, becoming similar to those generated in helped environments. These results may inform strategies for cancer vaccine dosing and schedules, as well as the inclusion of appropriate helper CD4+ T cell epitopes.
Write-up by Maartje Wouters, image by Lauren Hitchings




