With the goal of detecting and treating cancer as early as possible, Mascaux and Angelova et al. analyzed biopsies of premalignant and malignant lesions from patients who ultimately developed lung squamous cell carcinoma (SCC), and found that immune activation and subsequently immune escape occur in the pre-invasive stages of carcinogenesis. The results were recently published in Nature.
The researchers began by using gene-expression profiling to analyze 122 biopsies from 77 current and former smokers – the samples represented 9 morphological stages of lung SCC carcinogenesis, with the first 8 stages corresponding to normal tissue and premalignant lesions. Gene expression profiles grouped the 9 histologically identified stages into four steps of progression: normal bronchial tissue (stages 0-2: normal tissue and hyperplasia), low-grade lesions (stages 3-5: metaplasia, mild dysplasia, moderate dysplasia), high-grade lesions (stages 6 and 7: severe dysplasia, carcinoma in situ), and invasive SCC (stage 8).
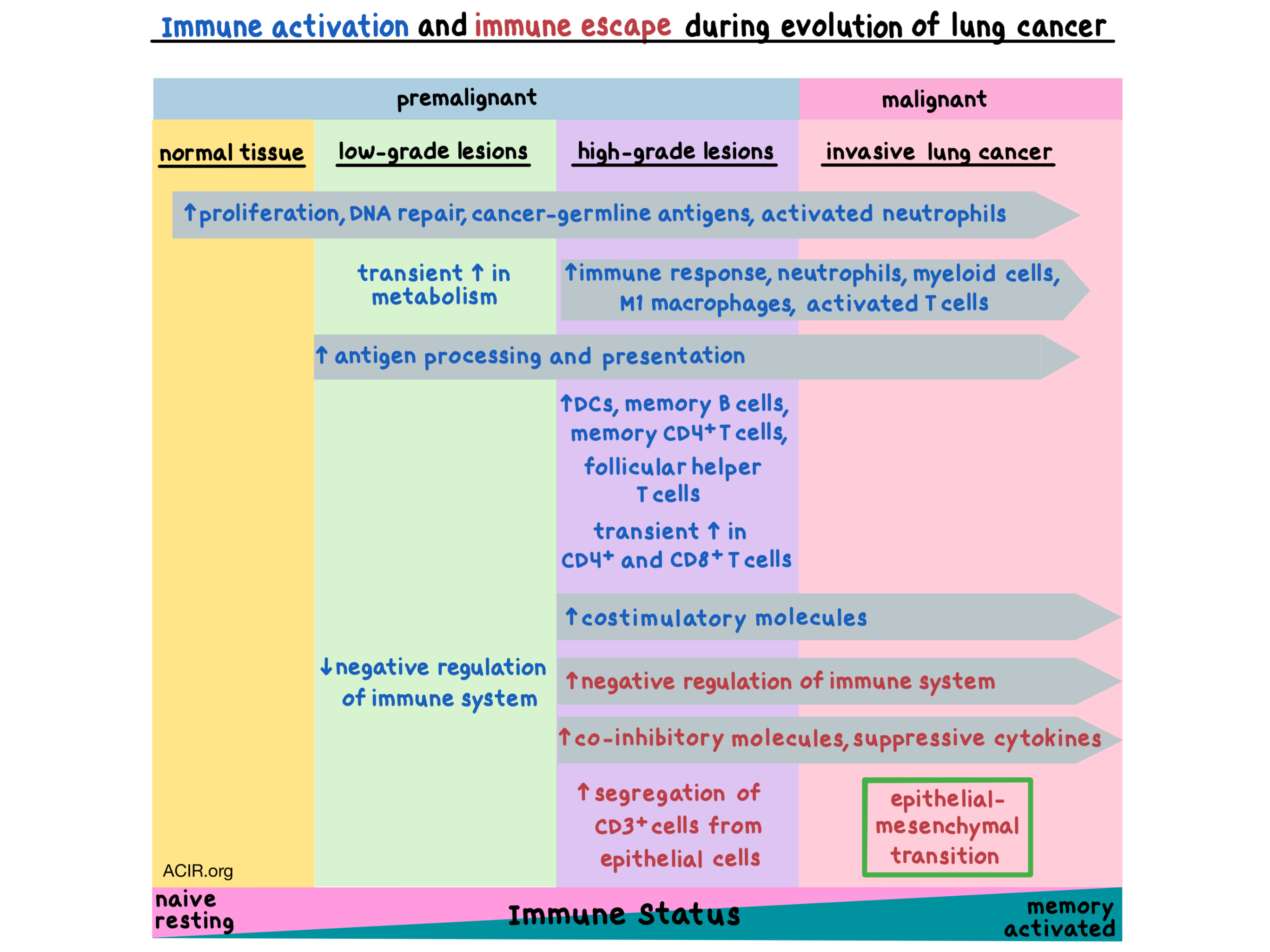
Gene expression analysis revealed that during the progression from normal tissue to cancer, the expression of genes associated with proliferation, cancer-germline antigens, and activated neutrophils increased starting from the earliest stages, while genes associated with downregulation of DNA damage response were decreased, indicating an increase in DNA repair with stage progression. The expression of genes involved in the immune response increased starting with the high-grade lesions, which also showed high expression of genes representing neutrophils, protumoral M1 macrophages, myeloid cells, and activated T cells. The expression of genes related to the epithelial-mesenchymal transition (e.g. CXCR4) increased only in the latest stage of invasive SCC, marking the onset of cancer. Metabolism genes exhibited a biphasic evolution, with the expression of genes related to fatty acid metabolism, oxidative phosphorylation, and citric acid cycle peaking in the low-grade lesions and then decreasing in the more advanced stages of carcinogenesis. Together, these results suggest that immune activation and sensing is present in the earliest stages of carcinogenesis.
Using gene expression profiles to estimate the abundance of various immune cell types, Mascaux and Angelova et al. observed an increase in myeloid-derived cells, neutrophils, macrophages, memory B cells, dendritic cells, follicular helper T cells, and activated CD4+ memory T cells in high-grade lesions. As the lesions progressed through the stages, the immune cells (including mast cells, B cells, NK cells, and CD4+ T cells) generally shifted from resting to activated and from naive to memory.
Functional analysis of differentially regulated genes in normal versus transformed tissues revealed that genes associated with negative regulation of the immune system were downregulated in low-grade lesions and upregulated in high-grade lesions and SCC, while genes associated with antigen processing and peptide antigen presentation were upregulated in all stages of transformed tissue. These data indicate that the immune response is activated early in the transformation process, but is suppressed as the lesions progress towards cancer.
Genes encoding co-inhibitory molecules (e.g. PDCD1, CTLA4, TIGIT, IDO1, PD-L1), suppressive interleukins (e.g. IL6, IL10, TGFβ), and other immunomodulatory molecules were expressed at increased levels in high-grade lesions and SCC. Genes encoding costimulatory molecules (e.g. 4-1BB, GITR, ICOS, CD80, CD86) were also upregulated in these later stages. Immunohistochemistry confirmed the statistically significant increase in the expression of immune checkpoints CTLA-4, IDO1, and PD-L1 at the protein level in SCC versus normal tissue. The increase in co-inhibitory and suppressive molecules in precancerous lesions indicates that immune escape occurs before tumor invasion.
The researchers then examined the microenvironment of the premalignant and malignant lesions using multispectral imaging and found differences in immune cell densities between the four stages of progression both in the epithelium and the stroma, with the two compartments demonstrating similar trends. CD4+ and CD8+ T cells were transiently increased in the high-grade premalignant lesions. Concordant with the gene expression data, myeloid, neutrophil, and macrophage densities increased in the high-grade lesions. PD-L1 and 4-1BB also increased in high-grade lesions, and even more so in SCC. Conversely, normal tissue and low-grade lesions had very few cells with 4-1BB, PD-L1, or FOXP3 phenotypes. Spatial characterization revealed that CD3+ cells were segregated from epithelial cells in all stages, but even more so in high-grade lesions, indicating reconfiguration of the tumor microenvironment.
Overall, Mascaux and Angelova et al. show that immune activation, surveillance, suppression, and escape occur in the pre-invasive, premalignant stages of carcinogenesis, supporting the use of immunotherapy as early in the treatment course as possible (possibly even as chemoprevention in patients at high risk of developing lung cancer) and highlighting the need for biomarkers that would enable early detection of cancer.
by Anna Scherer
Meet the researcher
This week, we interviewed Céline Mascaux, first co-author on this paper.

What prompted you to tackle this research question?
Lung cancer is the most deadly cancer and is diagnosed at a very late stage of the disease. Therefore, lung cancer research should focus on finding tools for its early diagnosis and its early treatment. Studying and understanding the first steps of lung carcinogenesis is thus essential for these purposes. We decided to collect precancerous lesions from current and former smokers and to dissect the mechanism of cancer development. The immune response appeared rapidly in our analyses as being a key phenomenon in lung cancer genesis.
What was the most surprising finding of this study for you?
We found that the role of the microenvironment and particularly the immune response is essential for the development of cancer at the earliest stage. Before the cancer invades the adjacent tissue, it already acquires several mechanisms of resistance against the host’s immune response. First, in low-grade precancerous lesions, we observed an immune sensing with a transient influx of naive T cells and downregulation of the genes that negatively regulate the immune system, among which we found TNFRSF14 (HVEM), CD200, CD59,TGFB3, and HLA-G. And then, the immune suppression mechanisms already occur in high-grade pre-cancer, by the increase of suppressive molecules (IDO1, CD274 (PD-L1), TIGIT, CTLA4, ICOS, IL10).
What was the coolest thing you’ve learned (about) recently outside of the lab?
Outside the lab, I am taking care of lung cancer patients. I really hope that we will translate these results into improvement of patient outcomes. We are planning a new project aiming to analyze the role of the immune process in the progression/regression of the precancerous lesions and I would like to propose chemopreventive approaches based on our findings.




