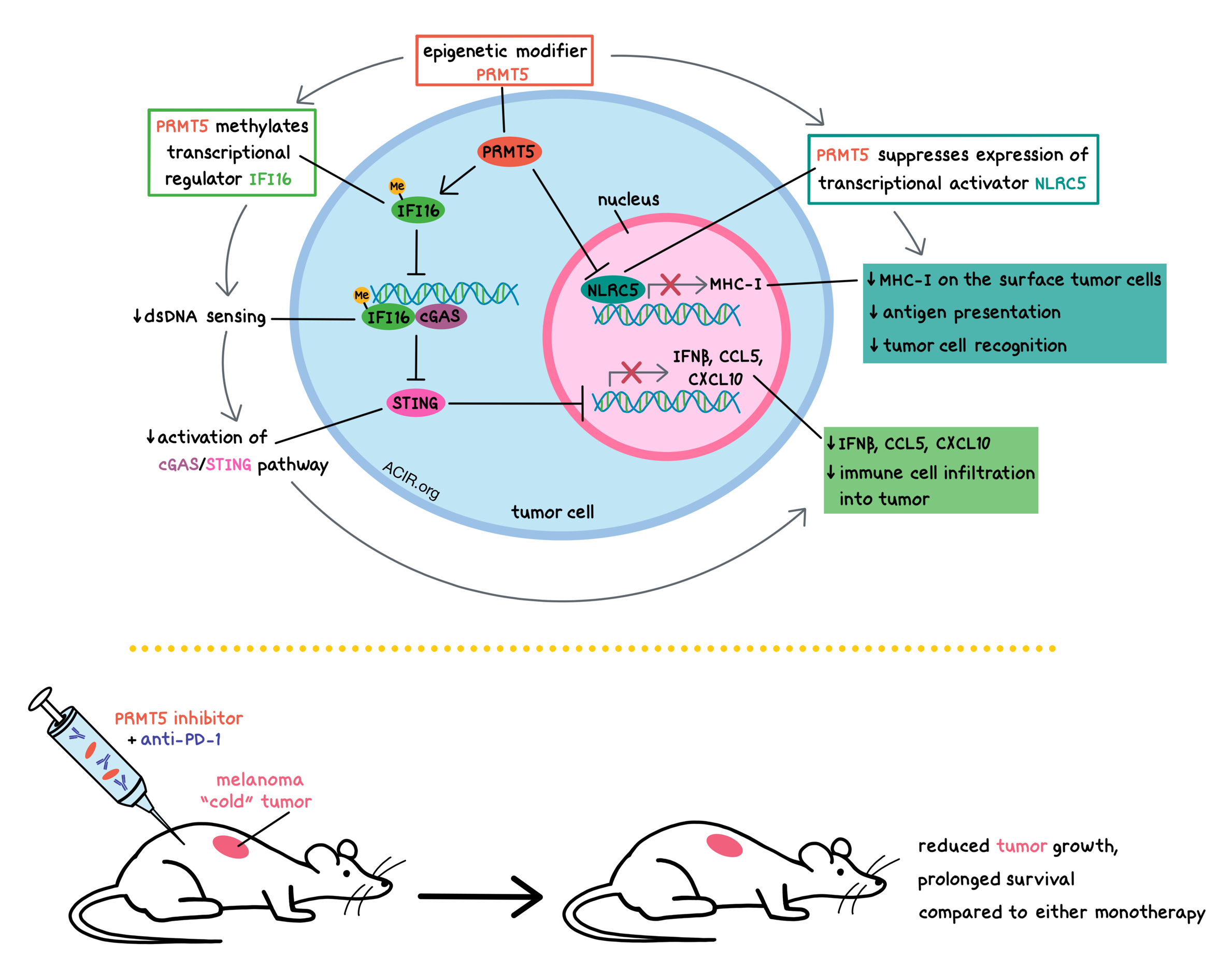
Aiming to more fully understand the mechanisms underlying the resistance of tumors to immune checkpoint blockade, Kim et al. explored the role that the epigenetic modifier PRMT5 (protein arginine methyltransferase 5), which regulates processes related to oncogenesis, may play in enabling immunosuppression in melanoma. The results, recently published in Science Translational Medicine, demonstrate that PRMT5 acts via two key immune mechanisms as an inhibitor of the antitumor response, and represents a potential therapeutic target.
The researchers began by analyzing melanoma patient datasets, particularly the subset of tumors with low expression of MTAP, as the accumulation of the MTAP substrate MTA inhibits PRMT5 and makes MTAP-deleted tumors more susceptible to PRMT5 inhibition. Within MTAP-low tumors, the researchers focused on the expression of SHARPIN, an adaptor protein that interacts with PRMT5 and plays a role in PRMT5 methylation of oncogene-related substrates. Consistent with prior work showing that both SHARPIN and MTAP increase PRMT5 activity, Kim et al. found that low SHARPIN expression in MTAP-low tumors was associated with better survival. Metastatic melanoma tumors with low expression of both MTAP and SHARPIN were enriched for genes associated with immune-related pathways (TH1, TH2, IL-2, Stat5,TNFα). Melanomas with low PRMT5 expression, or low expression of both PRMT5 and MTAP, showed similar immune response gene expression enrichment. PRMT5 is highly expressed in human melanomas, and its high expression was correlated with lower survival. These results suggested that PRMT5 plays a role in antitumor immunity.
In B16F10 metastatic murine melanoma cells, PRMT5 knockdown (KD) via short hairpin RNA (shRNA) decreased PRMT5 abundance and activity in vitro and in vivo; however, PRMT5 KD did not affect the growth of B16F10 cells in culture. In contrast, in immunocompetent C57BL/6 mice, inoculated PRMT5 KD B16F10 tumors exhibited significant reduction in tumor growth (up to 62% reduction in tumor volume and up to 54% reduction in tumor weight 17 days post inoculation) compared to controls. However, no reduction in tumor growth was observed in immunodeficient NSG mice. These observations suggest that PRMT5 KD-driven tumor growth inhibition relies on an intact immune system. To confirm this, the researchers showed that YUMM1.7 murine melanoma tumors with inducible PRMT5 KD also exhibited reduced tumor growth in C57BL/6 mice. Gain of PRMT5 function in tumor cells by ectopic expression increased tumor growth in immunocompetent mice, but not in immunocompromised mice. Together, these results suggested that PRMT5 promotes tumor growth by limiting antitumor immune responses via tumor-intrinsic mechanisms.
At day 17 post inoculation, a profile of intratumoral immune cells revealed a higher number of immune cells in PRMT5 KD tumors compared to controls, including CD4+ and CD8+ T cells, NK cells, dendritic cells (DCs), macrophages, myeloid-derived suppressor cells (MDSCs), and Tregs. The ratio of CD8+ T cells to MDSCs or to Tregs was significantly higher in PRMT5 KD tumors compared to controls, suggesting an antitumor immune phenotype. In contrast, tumors overexpressing PRMT5 and its adaptor WDR77 (which is essential for PRMT5 activity) had decreased immune cell infiltration. Depletion experiments demonstrated that the antitumor response and survival benefit conferred by PRMT5 KD was dependent on CD8+ T cells and NK cells.
Digging further into the mechanisms behind PRMT5 activity, the researchers performed an LC-MS/MS analysis of proteins interacting with SHARPIN, which revealed that the adaptor protein SHARPIN interacts not only with PRMT5, but also with IFNγ-inducible protein 16 (IFI16), which plays a role in p53 transcriptional activity and cell cycle regulation, and which is involved in cytosolic DNA sensing and interaction with cGAS/STING. In human A375 melanoma cells, inhibition of PRMT5 using the PRMT5 inhibitor EPZ015666 reduced IFI16 methylation in human melanoma cell lines and in B16F10 cells, and increased the interaction between IFI16 and SHARPIN. IFI16 binds to intracellular double-stranded DNA (dsDNA) and induces the expression of IFNB1 and chemokines CCL5 and CXCL10. PRMT5-dependent methylation of IFI204 (the murine homolog of IFI16) limited the activation of the cGAS/STING pathway, thus limiting the expression of IFNβ and the chemokines CCL5 and CXCL10. Together, these observations show that PRMT5 suppresses antitumor inflammatory responses via methylation of IFI16/IFI204.
Suspecting that PRMT5 suppresses antitumor responses in multiple ways, Kim et al. conducted a survey of metastatic melanoma datasets within The Cancer Genome Atlas and the Cancer Cell Line Encyclopedia for genes co-regulated with PRMT5, which revealed that expression of the transcriptional activator NLRC5 (NLRC5 plays a role in regulating MHC-I gene expression) was inversely correlated with PRMT5 expression. Further, the researchers showed that PRMT5 limited the abundance of MHC-I on the surface of melanoma cells by reducing the expression of NLRC5 and its target genes.
Tying together the two mechanisms by which PRMT5 suppresses the antitumor responses – suppression of inflammation by methylating IFI16, and reduction of MHC-I antigen presentation by reducing NLRC5 expression – the researchers observed that in vivo, expression of methylation-defective IFI204 and overexpression of NLRC5 in B16F10 cells mimicked PRMT5 KD and significantly inhibited tumor growth. The tumors had increased infiltration of CD4+ and CD8+ T cells, and also exhibited decreased expression of PRMT5, suggesting a possible feed-forward mechanism. In human melanoma specimens within TCGA, tumors with higher expression of either IFI16 or NLRC5 were associated with an enriched immune gene signature and prolonged survival of patients.
Relevant to clinical translation, PRMT5 KD tumors in mice not only had higher expression of Ifnb1, Ccl5, and Cxcl10, but also Pd-l1. Combination of PRMT5 KD (via shRNA) and anti-PD-1 significantly suppressed B16F10 tumor growth compared to anti-PD-1 alone, and significantly prolonged the survival of mice compared to either monotherapy. Combination of PRMT5 inhibitor GSK3326595 and anti-PD-1 conferred similar improvements in B16F10 and YUMM1.7 melanoma tumor growth reduction and prolonged mouse survival. Responses to GSK3326595 or anti-PD-1 monotherapies were limited. The antitumor response induced by the combination therapy was dependent on CD8+ T cells.
Overall, Kim et al. demonstrated that PRMT5 in melanoma tumors suppresses antitumor responses in two ways: 1) by methylating the transcriptional regulator IFI16, it reduces dsDNA sensing, limiting the activation of the cGAS/STING pathway, and therefore limiting the expression of IFNβ and the chemokines CCL5 and CXCL10; and 2) by suppressing NLRC5 expression, it reduces the abundance of MHC-I on the surface of melanoma cells, thus limiting antigen presentation and tumor cell recognition by immune cells. PRMT5 inhibition made “cold” tumors, which are not normally susceptible to immune checkpoint blockade, more responsive to anti-PD-1, suggesting potential clinical relevance for the combination treatment.
by Anna Scherer
Meet the researcher
This week, first author Hyungsoo Kim and lead author Ze’ev Ronai answered our questions.

What prompted you to tackle this research question?
We have previously studied PRMT5 and SHARPIN in melanoma and noticed their correlation with immune signaling pathways, using TCGA and other datasets. We thus set out to examine the possibility that PRMT5/SHARPIN may play a role in tumor immunity.
What was the most surprising finding of this study for you?
That PRMT5 controls two independent pathways that define tumor evasion from the immune system – the cGAS/STING (innate immunity) and the NLRC5 (antigen presentation) pathways. The complementary pathways controlled by PRMT5 in assuring effective immune evasion of melanoma were both surprising and assuring that PRMT5 does play a central role in effectiveness of antitumor immunity of melanoma.
What was the coolest thing you’ve learned (about) recently outside of work?
Expect the unexpected, and adjust. The changes in our life (and research) embraced by COVID-19, provided a new perspective to what we do, what is to cherish, and the adjustments one needs to make to secure scientific excellence and success going forward. Luckily, our work on the importance of PRMT5 on melanoma evasion from the immune system was not much affected since it was mostly completed before we encountered COVID-19, but going forward, we realize how we need to be mindful of time, resources, and the ability to perform critical experiments.




