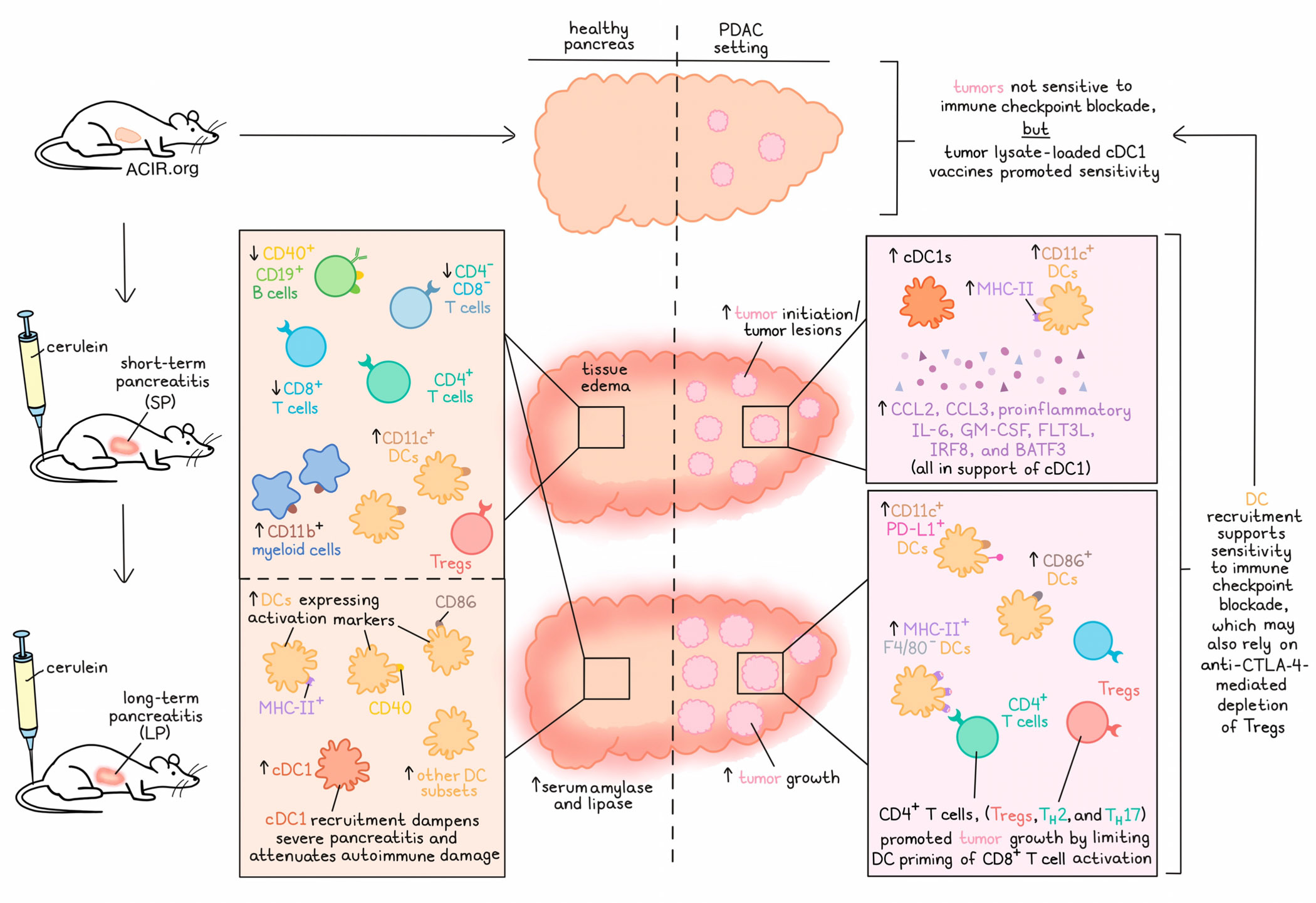
Pancreatic ductal adenocarcinoma (PDAC) remains refractory to immunotherapy due to a compromised tumor immune microenvironment (TIME) with dysfunctional T cells, suppressive myeloid and regulatory T cells (Tregs), and a sparsity of neoantigens and antigen-presenting cells. Patients with a history of chronic pancreatitis have an increased risk of developing PDAC, and progression of pancreatic cancer is often accompanied by pancreatitis. In a recent study published in Science, Mahadevan et al. conducted a context-dependent analysis of the PDAC tumor immune microenvironment and found that pancreatitis overlaying pancreatic cancer had an impact on antigen presentation and T cell immunity. Antigen-presenting cDC1s recruited by pancreatitis or administered as a vaccine sensitized PDAC to immune checkpoint blockade.
In mice, pancreatitis can be induced by short-term or long-term injection of the cholecystokinin analog cerulein. Short-term pancreatitis (SP) was characterized by tissue edema and more draining lymph nodes (dLNs), a decrease in frequencies of CD40+CD19+ B cells, double negative T cells, and CD8+ T cells, and an increase in CD11c+ (F4/80-Ly-6G-) DCs and various CD11b+ myeloid populations infiltrating the pancreas. Mice with SP further demonstrated a decrease in CD4+ and CD8+ T cells in dLNs and a global decrease in proliferating Ki67+ and Tbet+ CD8+ T cells, TH1 cells, and Tregs. Long-term pancreatitis (LP) was associated with increased serum levels of amylase and lipase, and phenocopied SP mice histologically and immunologically. The pancreas of LP mice harbored increased frequencies of cDC1s and other DC subsets expressing activation markers. In XCR-/- mice, which are characterized by systemic depletion of cDC1s, SP resulted in more severe pancreatitis with higher numbers of infiltrating CD4+Foxp3- T cells, suggesting that cDC1s attenuate autoimmune damage during pancreatitis.
SP accelerated tumor initiation in KC (Pdx1-Cre; LSL-KrasG12D/+) mice. At 10 weeks of age, 40-50% of the animals demonstrated pancreatic intraepithelial neoplasia (PanIN) lesions, whereas control KC mice showed similar levels of PanIN lesions only at 25 weeks of age. SP had no effect on the number of infiltrating CD4+ and CD8+ T cells, but led to a significant increase in cDC1s in PanIN lesions and associated dLNs in KC mice. CD11c+ DCs in PanINs expressed the activation marker MHC-II. scRNAseq data of SP KC pancreases revealed increased expression of CCL2, CCL3, proinflammatory IL-6, GM-CSF, FLT3L, IRF8, and BATF3, together required for the recruitment, development, activation, and maintenance of cDC1s.
To study the effect of pancreatitis on tumor progression, Mahadevan et al. induced LP in KPC (Pdx1-Cre; LSL-KrasG12D/+; Trp53R172H/+) mice bearing invasive tumors and observed accelerated tumor growth. The pancreas of LP KPC mice exhibited increased frequencies of CD11c+PD-L1+ DCs and CD11b+ myeloid cell subsets. The researchers also studied LP in iKPC* mice (WT mice orthotopically implanted with P48-Cre; LSL-KRASG12D/+; TRP53R172H/+ PDAC cell lines). In the pancreas of LP iKPC* mice, an increase in frequencies of MHC-II+F4/80-CD11c+ DCs and DCs expressing CD86 were observed, while myeloid cell subsets demonstrated no difference compared to control iKPC* mice.
After having observed an acceleration of PDAC initiation and progression in the setting of pancreatitis, the researchers dug deeper to study the effect of CD11c+ DC recruitment on T cell function. In contrast to KC mice with pancreatitis (SP or LP) or KC CD8-/- mice with pancreatitis, KC CD4-/- mice with pancreatitis had fewer PanIN lesions, and the number rebounded when CD8+ T cells or CD11c+ DCs were depleted (in SP KC CD4-/- mice). Similarly, SP KPC mice demonstrated accelerated PDAC progression and shorter overall survival than SP KPC CD4-/- mice. These findings suggested that CD4+ T cells in KC mice with pancreatitis, which were mainly Tregs, TH2, and TH17 cells, promoted tumor growth through suppression of a cytotoxic T cell response, either via inhibition of CD8+ T cells or CD11c+ DCs. IHC demonstrated increased spatial proximity of DCs and CD4+ T cells in LP KC mice and increased spatial proximity of DCs and CD8+ T cells in LP KC CD4-/- mice.
Mahadevan and team hypothesized that the pancreatitis-recruited and -activated DCs may also present tumor antigens and may render PDAC sensitive to immune checkpoint inhibitors. Treatment with anti-PD-1 or anti-PD-1 and anti-CTLA-4 inhibited tumor initiation in SP KC mice and eliminated PDAC in LP iKPC* and LP KPC-689 mice (WT mice orthotopically implanted with a KPC tumor-derived cell line), but not in mice without pancreatitis. The treated animals with pancreatitis revealed increased frequencies of CTLs; CD8+ T cell proliferation, activation, and memory; decreased Treg frequency; an increase in Tbet+ effector CD8+ T cells; and increased ratios of CD8/Treg and TH1/Treg, indicating that anti-CTLA-4 and/or anti-PD-1 may also relieve the suppressive effects of Tregs and thereby enable effector CD8+ T cell and TH1 responses. Similarly, administration of a vaccine consisting of tumor cell lysate-loaded CD103+ cDC1s, anti-PD-1, and anti-CTLA-4 resulted in complete tumor elimination in some animals with orthotropic PDAC (iKPC* and KPC-689) without pancreatitis. Treatment further led to a decrease in frequencies of CD4+PD-1+ and CD8+PD-1+ T cells, and an increase in CD3+CD69+ and CD8+CD69+ T cells. Tumor rechallenge experiments demonstrated immune memory in KPC-689 mice. In autochthonous KPC mice with a higher stromal component, the treatment scheme led to inhibition of tumor growth and longer survival.
The combination treatment resulted in a significant increase in frequency and CDR3 diversity of intratumoral CD8+ T cells in iKPC*. Twenty-four CDR3 sequences were enriched in these mice, and one sequence was identified in all of the mice that received the combination treatment.
Analysis of 120 PDAC samples obtained from treatment-naive patients revealed a significant correlation between tumor-infiltrating CD8+ T cells and CD11c+ DCs, as well as a weaker correlation between DCs and CD4+ T cells and Tregs. Patients with high levels of CD11c had a significantly longer disease-specific survival than patients with low levels of CD11c, while the number of CD8+ T cells or CD4+ T cells and Tregs did not predict survival. TCGA data analysis of 173 treatment-naive PDAC samples confirmed that high levels of cDC1s (identified via BATF3, XCR1, CLEC9A, and CADM1) correlated with significantly longer survival.
In sum, while in pancreatic cancer without pancreatitis, the majority of DCs were present within the dLNs, overlaying pancreatitis led to recruitment of activated DCs into PanIN and PDAC, where inhibitory CD4+ T cells suppressed the activation of a CTL response by the infiltrating DCs. PDAC with pancreatitis was sensitive to anti-CTLA-4 and anti-PD-1 immune checkpoint blockade (ICB), and the combination of a tumor cell lysate-loaded cDC1 vaccine with ICB demonstrated promising antitumor activity in various orthotropic and autochthonous PDAC mouse models. These findings may provide a new treatment approach for patients with PDAC.
Write-up by Ute Burkhardt, image by Lauren Hitchings




