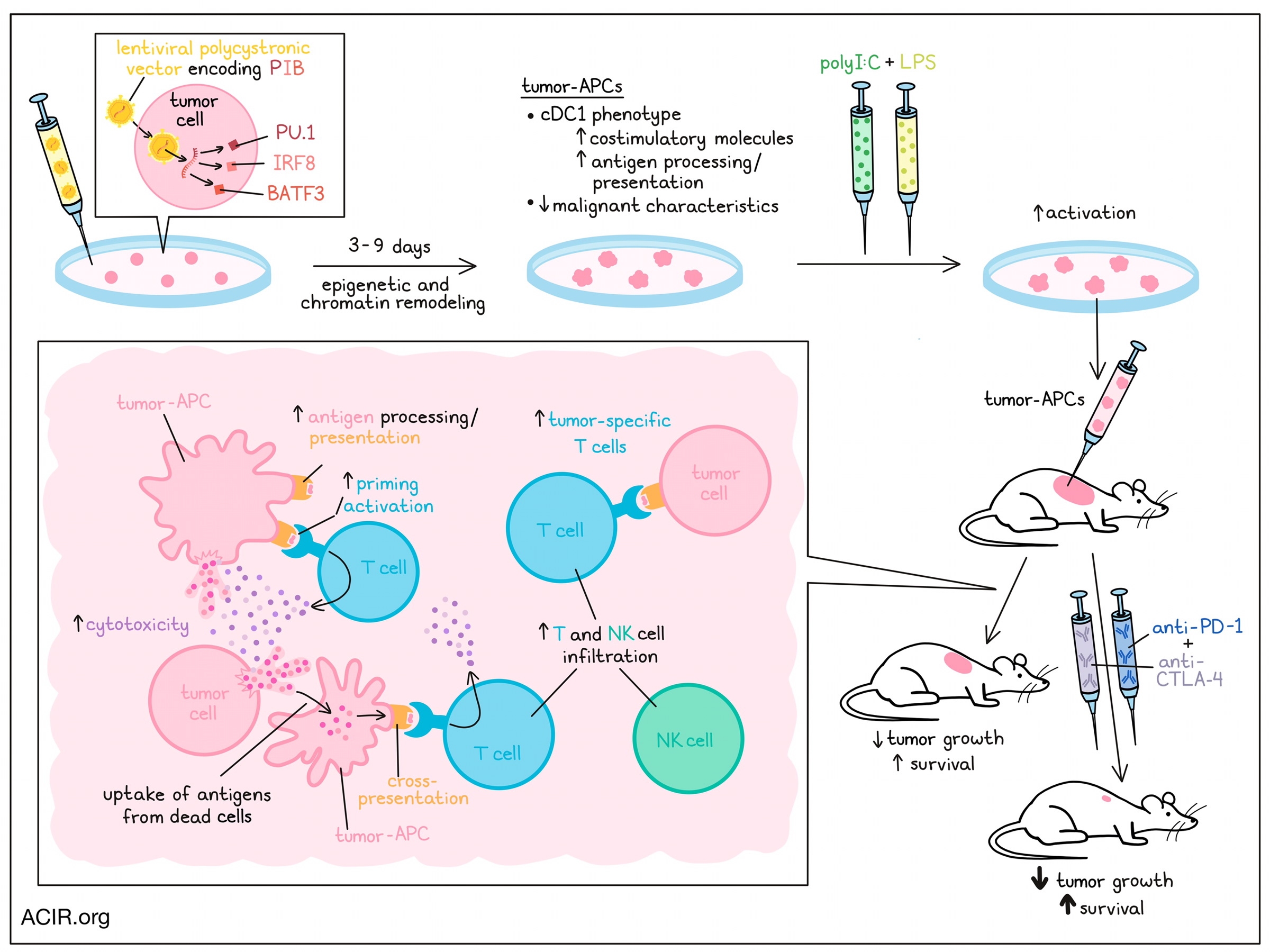
One immune evasion mechanism that limits antitumor immunity and response to immunotherapy is the downregulation of antigen processing and presentation in tumors. Zimmermannova and Ferreira et al. aimed to reverse this mechanism by forced expression of dendritic cell transcription factors in tumor cells to induce an antigen-presenting cell (APC) phenotype. Their results were recently published in Science Immunology.
To induce direct cell reprogramming, the researchers used a lentiviral polycistronic vector encoding mouse or human transcription factors PU.1, IRF8, and BATF3 (termed PIB). Having previously shown that PIB can reprogram fibroblasts into type 1 conventional dendritic cells (cDC1), the researchers tested PIB in he poorly immunogenic Lewis lung carcinoma (LLC) and B16F10 melanoma (B16) murine tumor lines. Transduced cells expressed CD45 and MHC-II, as well as the cDC1-specific marker CLEC9A, suggestive of a cDC1-like phenotype. Moving to human cells, the researchers used a panel of 28 solid tumor cell lines and 5 hematological cancer lines. Reprogramming with PIB induced a population of CD45+HLA-DR+ cells, which also expressed CLEC9A, CD226, and CD11c, and had a DC-like morphology, suggestive of induction of the cDC1 phenotype. These cells were therefore named tumor-APCs.
To assess gene expression changes after reprogramming, bulk RNAseq was performed on sorted reprogrammed cells. The reprogrammed cells showed a shift in transcriptome mapping that was similar to natural cDC1s, which allowed the creation of a human tumor-APC signature based on the cDC1 genes that were commonly upregulated in these lines during reprogramming. The upregulated genes in this signature were related to antigen processing and presentation, and immune interactions.
The reprogramming occurred fast, with some tumor-APCs arising as early as day 3 after transduction. To map the kinetics of the reprogramming, cells from the human glioblastoma T98G cell line were transduced with PIB-encoding lentivirus. When reprogrammed and partially reprogrammed tumor-APCs from various time points were subjected to RNAseq and ATAC sequencing, all time points clustered separately from parenteral cells, and the cells obtained at days 7 and 9 clustered closest to peripheral cDC1s. Epigenetic remodeling occurred mostly in the first three days, and chromatin remodeling was induced, as genes associated with cDC1 function had closed chromatin in parental cells, and open chromatin regions after reprogramming in transduced cells.
Mouse tumor-APCs had increased expression of β2-microglobulin and MHC-I, and human tumor-APCS had increased levels of HLA-ABC and HLA-DR, suggesting increased tumor immunogenicity. To determine if tumor-APCs could present tumor-associated antigens, the researchers applied mass spectrometry-based immunopeptidomics on B16-derived tumor-APCs. Tumor-APCs presented more peptides than controls, which were predicted to bind MHC-I, and included peptides that originated from known melanoma-associated antigens.
PIB transduction also induced costimulatory molecules on both mouse and human tumor cells. Coculture experiments of tumor-APCs (derived from B16 and LLC lines) and OT-1 cells showed that the tumor-APCs primed naive OT-1 CD8+ T cells. When B16-OVA tumor-APCs were cocultured with untreated B16-OVA cells with increasing ratios of activated OT-I cells, the tumor-APCs were more susceptible to T cell-mediated killing. At high T cell to target cell ratios, there was also killing of the non-target population, potentially caused by a bystander killing effect.
Further assessing the cDC1 function of tumor-APCs, the researchers found that the cells secreted IL-12p70, IL-29, CXCL10, and TNFα in response to Poly(I:C) and LPS. Tumor-APCs were also able to take up protein (OVA) and dead cells and process internalized antigens. Pulsing of naive OT-1 CD8+ T cells with the OVA peptide SIINFEKL while in coculture with tumor-APCs induced proliferation of the T cells. Tumor-APCs were also shown to cross-present antigens to CD8+ T cells in a coculture in which OVA protein was pulsed.
Zimmermannova and Ferreira and team then determined whether primary tumor cells could also be reprogrammed to tumor-APCs. To test this, samples were collected from patients with various solid cancer types. In all primary samples, CD45 and HLA-DR expression was induced in subsets of cells. Reprogrammed cells were purified and subjected to single-cell RNAseq. Most reprogrammed cells clustered closely to peripheral blood cDC1 and separate from parenteral cells. Reprogrammed cells had increased expression of cDC1 canonical markers, reprogramming markers, costimulatory molecules, endogenous IRF8 and BATF3, and the tumor-APC signature. Melanoma tumor-APCs were then stimulated with HLA-A2-restricted CMV pp65 and MART-1 peptides and cocultured with CD8+ T cells from HLA-A2+CMV+/MART-1+ donors. This led to an induction of memory CMV+CD8+ T cells and naive CD8+ T cells, similar to results obtained using monocyte-derived DCs.
Next, the tumor-APCs from primary melanoma were cocultured with tumor-infiltrating lymphocytes (TIL) from the same patient. After 8 hours, the TIL had increased expression of CD107a and CD137, and the cytokines IFNγ and TNFα, which correlated with increased cytotoxicity against the reprogrammed tumor cells. These TIL also had higher expression of BTLA, TIM3, LAG3, PD-1, CD28, and CD69. When anti-PD-1 or anti-CTLA-4 was added during the cultures, there was a small increase in cytolysis of the reprogrammed cells.
RNAseq of tumor-APCs showed that genes related to cell cycle progression and proliferation downregulated over time after reprogramming, and tumor suppressor genes were activated. In vitro experiments confirmed that reprogrammed cells had slower cell division than parental cells, and their colony formation was dramatically reduced. These data suggest that the reprogrammed cells lost some of their malignant characteristics.
Finally, in vivo experiments were conducted in syngeneic mice with B16-OVA tumors. The researchers generated B16-derived tumor-APCs, which were pulsed with OVA protein and stimulated with Poly(I:C), after which they were injected intratumorally. This treatment delayed tumor growth and prolonged survival. Increased numbers of tumor-specific T cells were detected in the blood and in tumor-draining lymph nodes (TDLN) after treatment. In the tumors, there was an increase in T and NK cells, including CD44+PD-1+ and CD44+PD-1- CD8+ and CD4+ T cells. Adding anti-PD-1 and anti-CTLA-4 treatment further reduced tumor growth and improved survival, suggesting a synergistic effect.
These data show that tumor cells can be reprogrammed into cDC1-like cells to activate tumor antigen presentation and overcome immune evasion mechanisms. The synergistic results seen in this study with immune checkpoint blockade suggest that this strategy might be a promising treatment in tumors with a “cold” immune environment.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the Researcher
This week, co-first authors Olga Zimmermannova and Gabriela Ferreira answered our questions.

What was the most surprising finding of this study for you?
We were initially surprised that the reprogramming process could be initiated in cancer cells from multiple tumor types, and that this process was extremely fast. However, we got most excited by the level of synergism that reprogrammed tumor antigen-presenting cells (tumor-APCs) reached in combination with immune checkpoint inhibitors in vivo. The game-changing moment was to see our first tumor-bearing animal being completely cleared of cancer with the tumor-APC reprogramming strategy. On the other hand, the most relieving moment was when we confirmed that reprogramming abolished tumorigenic properties in cancer cells.
What is the outlook?
In this study, we provided proof of principle that mouse and human cancer cells could be reprogrammed into antigen-presenting cells, and that reprogrammed tumor-APCs can initiate robust antitumor immune responses in vivo. The next step is to elicit the reprogramming of cancer cells in situ within tumors. We will place our efforts in translating our findings into an innovative cancer immunotherapy based on in vivo reprogramming of cancer cells. This spans from the development of systems allowing efficient delivery of the reprogramming factors into the tumors, preclinical safety studies, and the development of spheroid-based platforms to predict efficacy of tumor-APC based immunotherapy in patients.
What was the coolest thing you’ve learned (about) recently outside of work?
OZ: In general: We can smell petrichor (odor of soil after rain) better than sharks can smell blood. Concerning my summer plans: that you no longer need a visa to travel to Uzbekistan.
GF: There is such a thing as too much water for aloe vera. Learned it the hard way. Learned this week: Saturn's rings might be the result of a collapsed moon, and we are just lucky to live at the same time we can see them, because they might disappear over time.




