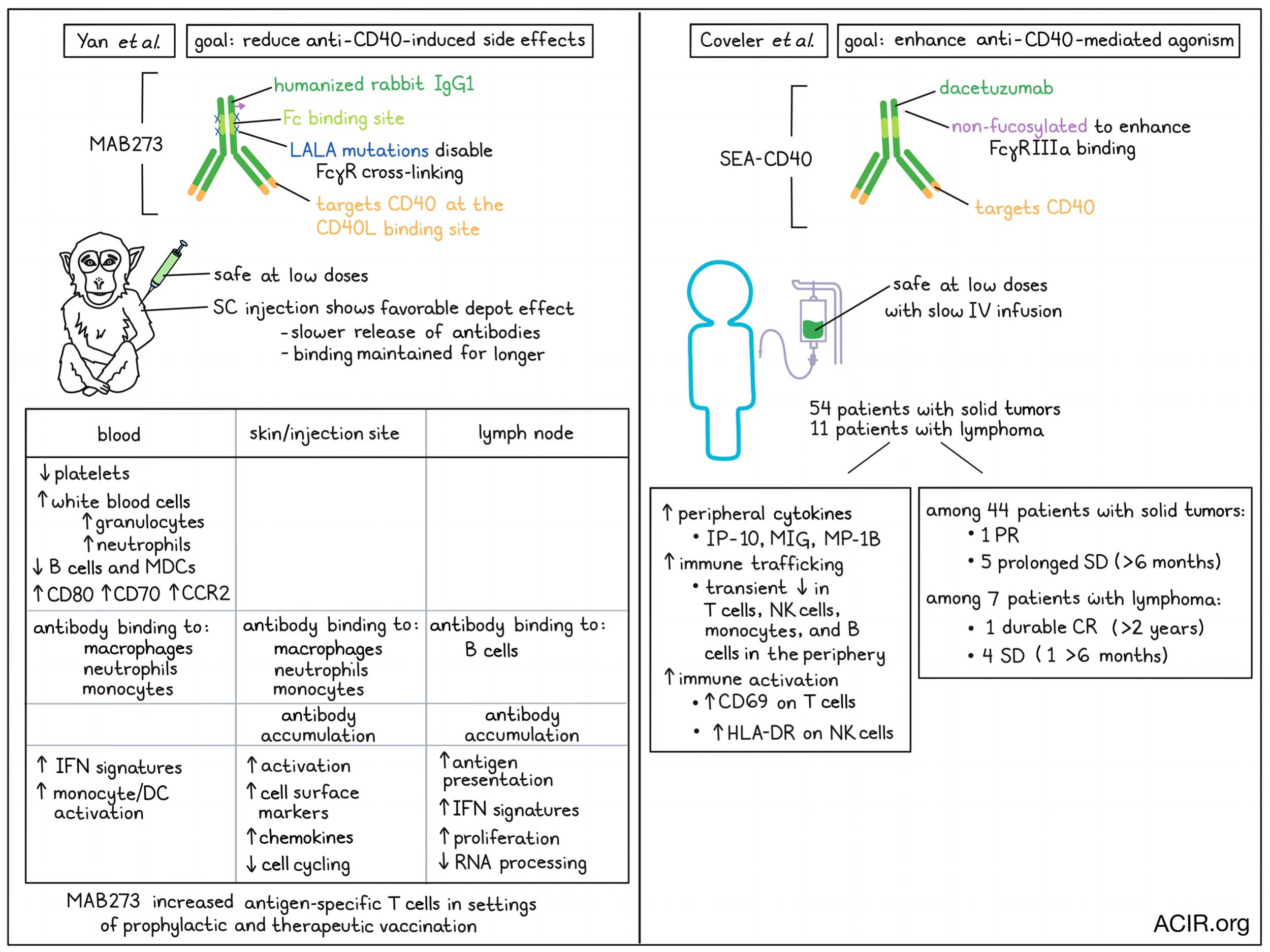
CD40 expressed on antigen-presenting cells (APCs) regulates immune responses by binding to CD40L on T cells, which stimulates antigen presentation and activation of both cell subsets. Therefore, targeting CD40 with an agonist antibody has potential as an immunotherapy for cancer. Current research into CD40 agonistic antibodies is focused on increasing efficacy and reducing toxicity. Two recent papers discussed the early development of two novel versions of CD40 antibody treatments. Yan et al. presented preclinical analyses of MAB273, an antibody with disabled Fcγ-receptor-mediated crosslinking, which was published in Cellular and Molecular Life Sciences. Coveler et al. reported Phase 1 study results of SEACD40, a non-fucosylated, Fcγ-receptor-binding CD40 agonistic antibody, in the Journal for Immunotherapy of Cancer.
With the objective of reducing the side effects of CD40 agonism, Yan et al. designed MAB273, a humanized rabbit IgG1 antibody with L234A, L235A (LALA) mutations in the Fc region to reduce Fcγ-receptor binding. In vitro experiments showed that this antibody specifically binds to the CD40L binding site, and that it can induce upregulation of the costimulatory markers CD80 and CD70 and the lymph node (LN) homing marker CCR7 on human B cells and myeloid dendritic cells (MDCs), as well as stimulate B cell proliferation.
To determine whether MAB273 prevents Fcγ-receptor mediated crosslinking, the researchers generated bivalent F(ab’)2 fragments (removing the Fc region while maintaining the hinge region), and monovalent Fab fragments (removing both the Fc and hinge regions). MAB273 and F(ab’)2 were similarly able to bind CD40 in a dose-dependent manner, while the Fab fragment showed weaker binding. MAB273 and F(ab’)2 both resulted in CD80, CD70, and CCR7 upregulation on B cells and MDCs, while the Fab fragment resulted in weaker activation of B cells. Therefore, these data suggest the effects of MAB274 are not dependent on FcγR crosslinking.
The researchers then assessed MAB273 in vivo in rhesus macaques with three administration groups (1 mg/kg intravenously [IV]; 0.1 mg/kg IV, and 0.1 mg/kg subcutaneously [SC]). The higher dose resulted in adverse events, while the lower dose was safe with both administration routes.
Generally, administration of MAB273 resulted in a rapid decrease in platelets and an increase in white blood cells, in particular granulocytes. There was also an increase in neutrophils and a decline in B cells and MDCs, which was dose-dependent. The plasma levels of IV MAB273 peaked early and declined gradually, reaching undetectable levels after two weeks. However, in the SC group, the levels of MABB273 were sustained without decline for a week, suggesting there was a depot effect with a slower release of the antibody into the blood.
Binding of CD40 in vivo on B cells and MDCs occurred rapidly after administration and was sustained for 72 hours for the high-dose IV group. In the SC group, this binding occurred later and lasted two weeks. As SC administration showed immune stimulation while also having a depot effect, this route may be less toxic, making it of interest for clinical development.
A biodistribution study with fluorescently-labeled, SC-injected MAB273 showed that most of the antibody was detected at the injection site and its draining LNs, while low or no levels were detected in further removed draining peripheral tissues. Various APCs bound MAB273; the most abundant subsets targeted with the antibody were macrophages, neutrophils, and monocytes, while in the draining LNs the B cells were the most targeted subset.
The researchers then performed RNAseq of draining LNs, injection site skin biopsies, and blood. Gene set enrichment analyses revealed that different gene modules were changed at the three sites studied. Most activation and changes were detected in the skin samples, with upregulation of genes associated with cell surface markers and chemokines, while cell cycle-related genes were downregulated. In the draining LNs, genes related to antigen presentation, interferon, and cell proliferation were upregulated, while genes related to RNA processing were downregulated. In the blood, genes associated with interferon signatures and monocyte and DC activation were upregulated.
Finally, the value of adding MAB273 as an adjuvant to vaccination was determined in six rhesus macaques. One group received seven HIV-1 envelope glycoprotein (Env) peptides twice to establish low levels of immunity, after which they were boosted with MAB273 together with the peptides to mimic therapeutic vaccination. Another group received MAB273 plus Env peptides in a prime-boost schedule to mimic prophylactic vaccination. In the therapeutic setting, low frequencies of specific T cells were detected after Env peptide immunization alone, which increased upon the boost with peptides and MAB273. In the prophylactic setting, higher levels of T cell responses were detected at prime immunization as compared to Env alone. Subsequent boost immunizations reactivated the T cells, but these did not reach the peak levels achieved by the initial priming immunization. These data suggest that MAB273 might improve vaccine responses in the cancer setting.
Focusing on enhancing CD40 agonism, Coveler et al. generated a non-fucosylated version of the CD40-agonizining antibody dacetuzumab to increase binding capacity to FcγRIIIa. With this, they performed a phase I, dose-escalating trial (IV delivery), including 54 patients with solid tumors and 11 patients with lymphomas. Most of the adverse events (AEs) that patients experienced were grade ≤3, with infusion-related reactions being the most common. Two patients had grade 4 AEs; one case with acute myocardial infarction and one case with infusion/hypersensitivity reactions (IHRs). IHRs were commonly reported, which was found to be associated with the infusion rate. Therefore, a standardized slow infusion approach was implemented. A dose of 30 μg/kg was found tolerable, while higher doses were intolerable due to IHRs, even with slow infusion.
The infusion of SEA-CD40 increased cytokines in the periphery in a dose-dependent manner, in particular, interferon-γ-inducible protein-10 (IP-10), monocyte chemoattractant protein-1, monokine induced by interferon-γ (MIG), and macrophage inflammatory protein-1b (MIP-1b). The cytokine increases were observed between 4-24 hours after infusion and normalized within 24-168 hours.
Treatment also induced immune activation and trafficking, with rapid decreases in T cells, NK cells, and monocytes in the first four hours after infusion. T cells normalized after 24 hours and NK cells and monocytes after 72 hours. There was also a dose-dependent reduction in B cells. Increased activation was shown by induced CD69 expression on T cells and HLA-DR on NK cells.
Out of 44 analyzable patients with solid tumors, 6 had a reduction in tumor burden after treatment. This included one partial response and five prolonged stable disease cases of ≥6 months. Of the seven patients analyzable with lymphomas, one had a durable complete response, which was maintained for >2 years, and four patients had stable disease, and one of these lasted >6 months.
Together, these data suggest that variations of CD40 antibodies can induce immune activation, with a relatively good safety profile. It will be of interest to assess combination therapy with other immunotherapeutics to further enhance the antitumor immune response.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author Andrew Coveler and lead author Juneko Grilley-Olson answered our questions.

What was the most surprising finding of this study for you?
This study demonstrated the safety and feasibility of administering a CD40 agonist. It successfully stimulated the immune system, but this did not clearly yield a response.
What is the outlook?
The next step was and is to consider combination therapies, such as in pancreatic cancer.
What was the coolest thing you’ve learned (about) recently outside of work?
AC: I learned there is a green heron!
JGO: In the bird theme, I recently learned that pigeons make milk for their chicks, from watching Wild Kratts with my kid.




