In clinical practice, radiation oncologists frequently perform elective nodal irradiation (ENI) to irradiate clinically uninvolved tumor-draining lymph nodes (TDLN) in addition to the localized tumor in order to preemptively treat potential nodal micrometastases. However, it is unclear how the irradiation of TDLN affects adaptive immune response and the efficacy of radiation therapy in combination with immune checkpoint blockade (ICB). Marciscano et al., in their recent paper published in Clinical Cancer Research, attempted to answer this question by developing a preclinical model of ENI and comparing the immunological effects of tumor-only stereotactic radiotherapy alone (RT) and tumor radiotherapy + ENI (RT + ENI), as monotherapies or in combination with ICB.
To develop their preclinical model, the team utilized cone-beam computed tomography imaging using the small animal radiation research platform (SARRP) on C57BL/6J mice with implanted B16 melanoma or MC38 colorectal carcinoma to perform image-guided stereotactic radiotherapy on the tumor or the tumor and the TDLN. The absence of micrometastatic disease in the TDLN at the time of radiotherapy was confirmed by a pathologist.
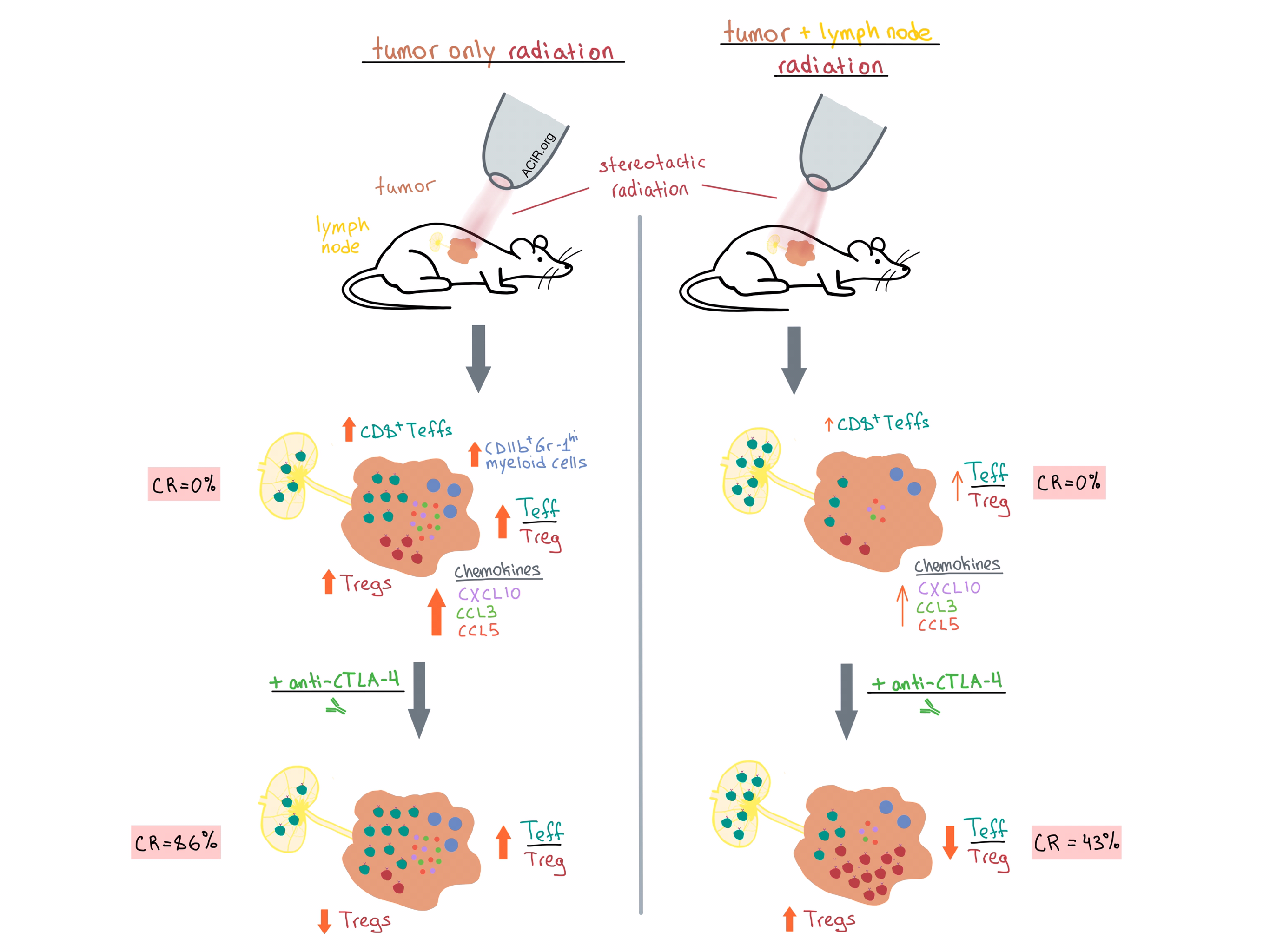
Marciscano et al. irradiated mice 11 days after tumor implantation and found that RT significantly increased the tumor infiltration of CD8+ effector T cells (Teffs), Tregs, and immunosuppressive CD11b+Gr-1hi myeloid cells, while RT + ENI led to only a slight increase in Teffs and did not significantly affect the other two cell subsets. Both treatments increased the CD8+ Teff/Treg ratio relative to non-irradiated controls, although the effect was significantly greater with RT than with RT + ENI. The increased intratumoral infiltration of Teffs following RT compared with RT + ENI was also confirmed via adoptive transfer of OVA-specific CD8+ T cells (OT-1) into mice with OVA-expressing tumors.
Digging deeper into the mechanism behind the differential immunological effects of the two radiotherapy treatments, Marciscano et al. observed that RT led to upregulation of intratumoral CXCR3- and CCR5-associated chemokines (CXCL10, CCL3, CCL5, etc.), which play a role in recruitment of CD8+ Teffs into the tumor microenvironment (TME). Chemokine upregulation was lower after RT + ENI, which may explain the lower Teff accumulation in the TME following this treatment. It is worth noting that RT + ENI did not negatively affect T cell priming within the TDLN. In fact, adoptive transfer experiments demonstrated that RT + ENI resulted in significantly higher proportion of cytokine-producing OT-1 cells in the TDLN compared with RT. However, this increase in TDLN Teffs did not translate to increased migration into the tumor due to decreased chemokine expression.
Next, the team sought to examine whether RT and RT + ENI differentially impact survival when used as monotherapy or combined with ICB. As monotherapies, both RT and RT + ENI prolonged survival (41 and 25 days, respectively) compared with control, but neither therapy led to complete tumor regression or long-term survival. Combining either radiotherapy strategy with anti-PD-1 improved survival compared with anti-PD-1 alone (~45 days versus 25 days) and led to a complete response rate of approximately 30% for both RT/anti-PD-1 and RT + ENI/anti-PD-1. Anti-CTLA-4 monotherapy, which partially depletes Tregs, resulted in 29% complete tumor regression rate and median survival of 44 days. Combination RT + ENI/anti-CTLA-4 further improved the outcome, with survival of 62 days and complete response in 43% of mice. However, the best observed outcome was with RT/anti-CTLA-4 treatment, which resulted in complete response rate of 86% and median survival rate that had not been reached by day 90.
Using immunophenotyping to quantify the differences in immunological effects of the different treatment groups, the researchers found that the absolute number of CD8+ Teffs and the Teff/Treg ratio in the tumor positively correlated with survival. Not surprisingly, the RT/anti-CTLA-4 treatment group demonstrated the highest Teff/Treg ratio and CD8+ Teff infiltration in the tumor. Interestingly, while anti-CTLA-4 decreased intratumoral Tregs when used as monotherapy or in combination with RT, combining anti-CTLA-4 with RT + ENI increased intratumoral Tregs, negatively impacting survival.
Surviving mice (found only in combination treatment groups) were rechallenged six months after initial tumor implantation to examine the formation of memory response. Mice treated with anti-CTLA-4 in combination with either radiation strategy had a more robust memory response than mice treated with anti-PD-1 in combination with either radiation treatment (100% versus 50%, respectively). Stratifying memory response by radiation type (combined with either ICB) revealed no significant difference in survival. Together, these results indicate that while the choice of radiotherapy treatment impacts initial response and survival, long-term protection is more dependent on the choice of ICB.
Overall, Marciscano et al. demonstrate that irradiation of the clinically uninvolved TDLN attenuates the immunomodulatory benefit of stereotactic irradiation of the tumor, negatively impacting adaptive immune response via reduced chemokine expression and reduced trafficking of CD8+ Teffs into the tumor. Moreover, synergy of tumor irradiation with anti-CTLA-4 is abrogated. The preclinical data presented in this study suggests that irradiation of TDLN should be carefully reconsidered in order to improve the efficacy of radiotherapy/immunotherapy combination treatment in the clinic.
by Anna Scherer




