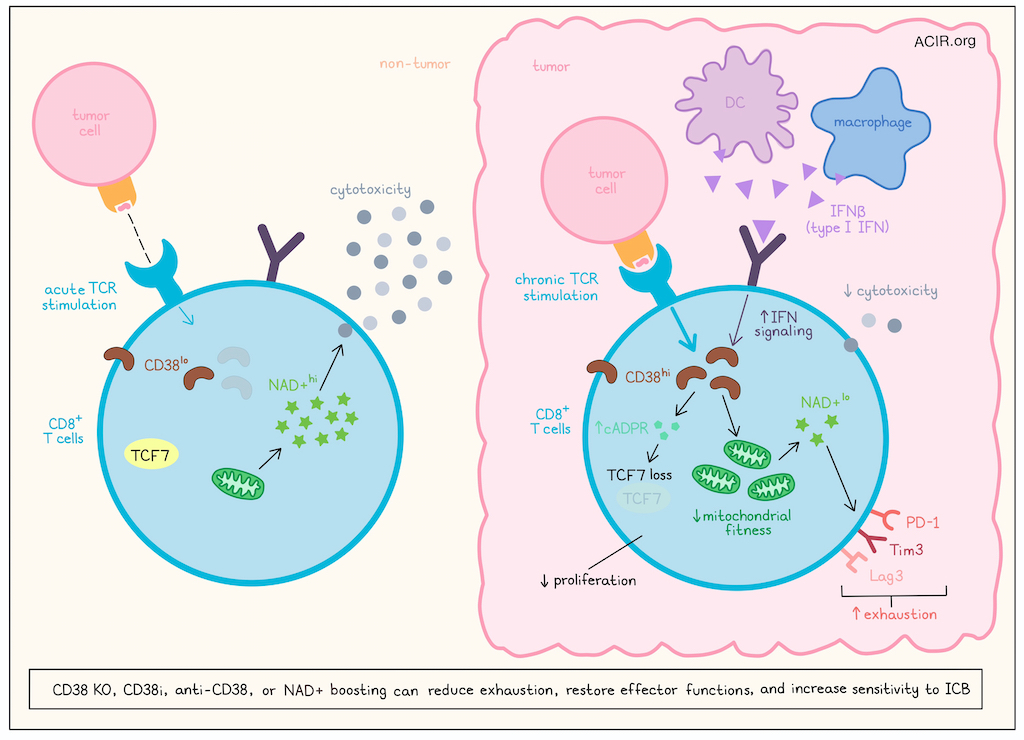
Immune checkpoint blockade (ICB) can unleash immune responses against a variety of cancers, but its efficacy is often limited by T cell exhaustion. In addition to well established checkpoints like PD-1 and CTLA-4, T cell exhaustion has also been linked to CD38 and NAD+. In recent work published in Cell Reports Medicine, Revach et al. explored this axis and the potential of targeting it to limit T cell exhaustion and improve ICB.
To begin, Revach et al. used publicly available scRNAseq datasets from patients with advanced melanoma treated with ICB, and found that CD38 expression was increased in exhausted CD8+ TILs and decreased in active or effector memory T cells, which expressed higher levels of TCF7. Further, a higher proportion of CD8+ TILs expressing CD38 were identified in patients that were resistant to ICB (ICB-NR; both at baseline and to a greater extent post-treatment) than in those responsive to ICB (ICB-R). Further, high CD38 expression could be used to predict resistance to both anti-PD-1 and anti-PD-1 plus anti-CTLA-4. Similar results were noted in an independent cohort and in a cohort of patients with NSCLC.
Next, the researchers generated time-resolved scRNAseq data from murine B16 melanoma, tumor draining lymph nodes (dLNs), and normal lymph nodes (nLNs), and found that Cd38 expression was evident in CD8+ TILs by day 7 and increased over time with increasing tumor burden. Meanwhile, Tcf7 expression was observed largely in CD8+ T cells in dLNs and nLNs, and was lost in CD8+ TILs. In B16-OVA tumors treated with anti-PD-1, Cd38 expression was increased only in terminal exhausted/effector CD8+ TILs. Looking at peripheral blood from patients with melanoma before and after ICB treatment, the researchers identified higher levels of CD38+CD8+ T cells in ICB-NR patients mainly after treatment. CD38 was also increased in the plasma in both ICB-R and ICB-NR patients at 6 weeks, but remained elevated long-term only in ICB-NR patients.
In data from patients with melanoma, the researchers found that CD38 was generally expressed alongside multiple co-inhibitory receptors, and similar patterns were seen in TILs from B16-OVA. Similarly, comparing CD38hi and CD38lo B7-H3-directed CAR T cells, the researchers found that CD38-high cells had increased expression of exhaustion-related surface markers and decreased expression of TCF7.
To model the development of dysfunction in CAR T cells, the researchers showed that chronic TCR stimulation increased CD38 expression, reduced proliferation, increased exhaustion marker expression, and reduced lysis of target cancer cells compared to acute stimulation. Similar results were observed in patient-derived CD8+ TILs cultured with matched primary tumor cells. Using CRISPR-Cas9, Revach et al. showed that cells deficient in CD38 had a proliferative advantage following chronic TCR stimulation, with an increased proportion expressing TCF7 and showing cytotoxicity towards patient-derived melanoma cell lines.
Next, the researchers investigated how CD38 relates to exhaustion by using CD8+ TILs from ICB-treated melanoma patients and CD3+ TILs from B16-OVA tumors. In these cells, genes related to type I/II IFN signaling and OXPHOS were enriched, suggesting differences in mitochondrial fitness. Upon further investigation, the researchers found evidence that mitochondrial fitness was reduced in CD38hi versus CD38lo B7-H3 CAR T cells and CD8+ TILs from human melanoma, and that CD38 deletion improved mitochondrial fitness. As NAD+ boosting has also been shown to restore mitochondrial fitness, the researchers demonstrated that chronically TCR-stimulated B7-H3 CAR T cells demonstrated decreased levels of NAD+, and that deletion or inhibition of CD38 restored NAD+ pools, reduced expression of exhaustion markers, and increased expression of TCF7. Further, NAD+ depletion induced a more exhausted state, while NAD+ boosting increased T cell functionality without affecting CD38 expression. However, the effect of NAD+ on TCF7 expression was limited, so the researchers investigated other pathways, and found that CD38 also promoted expression of cADPR, which regulated expression of TCF7 via the ADPR/Ca2+/RyR2/AKT pathway.
To better understand the mechanism underlying the increase in CD38 expression in exhausted TILs, the researchers evaluated the association between CD38 and type I/II IFN signaling. Analysis of scRNAseq data of CD8+ TILs in human melanoma revealed co-expression of CD38 and several type I IFN-stimulated genes (ISGs) in exhausted T cells, with higher expression of ISGs in T cells from ICB-NR patients. Further, high expression of IFN signaling machinery in macrophages and DCs was associated with resistance to ICB, leading the researchers to hypothesize that IFNs in the TME could induce the expression of CD38 and drive T cell dysfunction. Testing this, the researchers found that CD8+ TILs expanded from melanoma patients upregulated CD38 expression when treated with IFN-β (type I IFN) – but not IFNγ (type II IFN) – and this upregulation was increased further when IFN-β was combined with TCR activation. Treatment with IFN-β also induced features of exhaustion similar to those in CD38hiCD8+ T cells. A JAK1/2 inhibitor prevented this upregulation of CD38, confirming the role of IFN sensing in T cell-specific upregulation of CD38.
To investigate the effects of CD38 on resistance to anti-PD-1, the researchers utilized melanoma PDOTS – short-term 3D cultures of multicellular spheroids derived from freshly explanted patient tumors, retaining tumor-infiltrating immune and stromal cells, including CD8+ TILs. Treatment of PDOTS with anti-PD-1 plus anti-CD38 was more effective than either monotherapy, with strong ex vivo responses (monitored by tumor cell viability) to the combination even in samples derived from patients that were clinically resistant to ICB. Proliferating CD8+ T cell and CXCL13+ T cell clusters showed the highest expression CD38, and in PDOTs that were responsive to dual-blockade, cells in these clusters showed increased expression of genes associated with effector functions and cytotoxic potential. Module scores of these gene sets could predict responding from non-responding PDOTS with 85% accuracy. Similar results were observed in MDOTS derived from murine B16-OVA or CT26-GFP, which were also shown to require CD8+ T cells for the antitumor efficacy of dual blockade. Finally, the researchers investigated whether CD38 inhibition (CD38i) or restoring NAD+ could overcome CD38-mediated exhaustion and found that indeed, in MDOTS, both strategies enhanced responses to anti-PD-1. Further, NAD+ depletion blunted the effects of anti-PD-1 plus anti-CD38, underscoring its role.
Overall, these results show that exposure to chronic TCR stimulation and increased IFN-I in tumors drives increased expression of CD38 in CD8+ T cells, which in turn, reduces mitochondrial fitness and depletes NAD+, driving exhaustion and contributing to resistance to ICB in melanoma and other cancers. Targeting CD38 or supplementing NAD+ could potentially overcome these effects and sensitize tumors to ICB.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Or-Yam Revach and lead author Russell W. Jenkins answered our questions.

What was the most surprising finding of this study for you?
CD38, an ecto-enzyme involved in NAD⁺ catabolism, is highly expressed in exhausted CD8⁺ T cells and has emerged as a promising target to enhance immune checkpoint blockade (ICB) by blunting T cell exhaustion. However, its efficacy in human cancer remained unclear. Using ex vivo profiling of a cohort of patient-derived organotypic tumor spheroids (PDOTS), we were amazed to see over 50% of tumors respond to CD38/PD1 blockade combination, compared to only 7.4% to PD-1 alone. Even more striking was that half of these responders were ICB resistant tumors clinically, suggesting that targeting CD38 could both enhance responses and overcome resistance.
What is the outlook?
In our study, we repurposed daratumumab, an FDA-approved anti-CD38 antibody for multiple myeloma, to restore ICB sensitivity by targeting T cell exhaustion in melanoma. The striking response observed in PDOTS to combined PD-1/CD38 blockade supports rapid clinical translation using existing antibodies. While prior trials of CD38/PD-(L)1 blockade in some solid tumors where CD38 targeting was directed at the cancer cells showed limited efficacy, they didn’t reveal major safety concerns. Thus, there is a clear path to rapid translation of the CD8-specific targeting of CD38 in combination with ICB in refractory melanoma.
What was the coolest thing you’ve learned (about) recently outside of work?
I’m a mother of two and a scientist. During my postdoctoral training, my kids reached the age where they became curious about my work and even asked why I haven’t solved the cancer problem yet! Their innocent, thoughtful questions have been surprisingly inspiring. They push me to see problems from fresh perspectives and often spark ideas or solutions I might not have considered otherwise.




