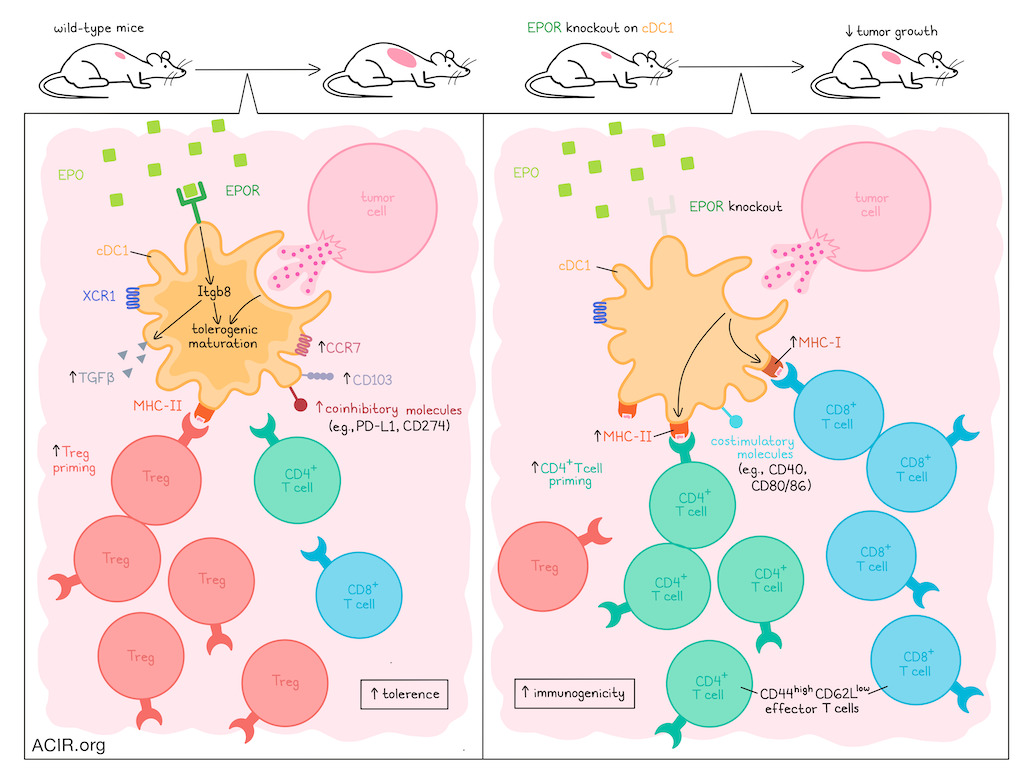
The priming of an immune response is a complex process that is largely dependent on the functional state of cDC1s, which can induce either immunogenic or tolerogenic priming, depending on a number of conditions. While numerous factors associated with antigen uptake and presentation have been linked to different immune outcomes, the mechanisms that determine how cDC1s become tolerogenic are not well understood. In recent research, Zhang et al. investigated these mechanisms and identified erythropoietin receptor (EPOR) on cDC1s as a critical regulator.
To begin, Zhang et al. used an established protocol combining total lymphoid irradiation (TLI), anti-thymocyte serum (ATS), and allogeneic donor bone marrow infusion to induce mixed chimerism and donor-specific tolerance to fully MHC-mismatched transplanted organs in mice. In this model, tolerance was dependent on cDC1s, which were increased among splenic cDCs, expressed XCR1, and showed increased signs of proliferation, phagocytosis, apoptotic cell clearance, erythropoiesis, and myeloid cell migration. They also expressed increased levels of EPOR, particularly among mature (CCR7+) cDC1s, and this was accompanied by increased CD103 expression (associated with tissue residency) and changes in metabolism.
Following the hypothesis that EPO–EPOR signaling in cDC1s may contribute to a tolerogenic state, the researchers generated mice in which EPOR was deleted in cDC1s. In these mice, TLI/ATS-induced bone marrow chimerism was lost, and mice rejected transplants. In vitro, the researchers showed that supplementing with EPO enhanced antigen-specific Treg induction by wild-type cDC1s, while deletion of EPOR reduced Treg induction. Similar effects were observed in vivo.
Given that tolerogenic maturation can be induced by efferocytosis in splenic cDC1s in the homeostatic state, the researchers evaluated untreated mice, and identified a wide range of tolerogenic maturation states. Analysis of TLI/ATS treatment then showed increases in both early and late-mature cDC1s, increased efferocytosis-related genes, enhanced tolerogenic maturation, and reduced cross-presentation to both CD4+ and CD8+ T cells. These effects were attenuated in mice in which EPOR was knocked out on cDC1s, further supporting the role of EPOR in tolerogenic maturation.
Comparing the transcriptional profiles of EPOR+ and EPOR- cDC1s, the researchers identified unique gene sets. EPOR+ cells spanned the spectrum of tolerogenic maturation, but were biased towards a more mature phenotype, while EPOR- cDC1s showed a profile consistent with more immunogenic, immature phenotypes, expressing lower levels of XCR1, CCR7, and coinhibitory receptor CD274, even without TLI/ATS treatment.
Looking into the mechanism of EPOR-induced cDC1 tolerogenic maturation, the researchers identified increased expression of Itgb8, Scube3, Tgfb1, and Ccl22 – genes associated with Treg induction and maintenance – in CCF7+ late mature cDC1s after TLI/ATS, dependent on EPOR expression. Further investigation revealed that Itgb8 and its activation of latent TGFβ were required for Treg induction.
Next, the researchers investigated how EPOR expression in cDC1s impacts priming of CD8+ and conventional CD4+ T cells, and found that without EPOR expression, cDC1s expressed significantly higher levels of CD40, CD80, MHC-I, DEC205, and Bcl-XL (anti-apoptosis), and lower levels of PD-L1, even in CCR7- cDC1s. While frequencies of splenic CD8+ and conventional CD4+ and CD8+ T cells were similar, EPOR deletion in cDC1s increased CD44hiCD62Llow effector phenotypes in both subsets. In an OVA model system, mice with cDC1s lacking EPOR showed enhanced priming of antigen-specific CD8+ and CD4+ T cell, while in wild-type mice, exogenous EPO enhanced Treg generation among CD4+ cells, suggesting that EPOR limits CD8+ T cell cross-priming, and favors Treg priming over priming of conventional CD4+ T cells.
To determine where this effect was induced, the researchers assessed migratory cDC1s, along with lymph node cDC1s in peripheral and mesenteric lymph nodes (PLNs and MLNs; mesenteric lymph nodes drain primarily from the gut). In PLNs, about 7% of cDCs expressed EPOR. Nearly all EPOR+ cells were migratory, and most were cDC1s. Meanwhile EPOR+ migratory cDCs were largely absent in MLNs. These results suggested that cDC1s acquired EPOR expression in the periphery, prior to migration to DLNs. Further, when apoptotic cells were injected into mammary fat pads and local DC1s were tracked, the majority of cDC1s were found to express EPOR and CD103, with evidence of enhanced engulfment and increased induction of Tregs. These effects could be further enhanced with systemic EPO administration, and were abrogated in mice with EPOR deleted from cDC1s. Similar results were observed in a chimeric OVA-expressing ACT model.
Looking at scRNAseq data for migratory DCs, the researchers found that when EPOR was knocked out in cDC1s, clusters characterized by high Itgb8 expression or immunoregulatory markers were significantly reduced, while a cluster with a gene profile for CD4+ T helper licensing was enriched. Flow cytometry showed upregulation of CD40 and CD86, though PD-L1 levels remained the same. EPOR loss also increased expression of genes involved in MHC-II-mediated antigen presentation, cross-presentation, cytotoxic T cell responses, costimulation, immunogenic maturation, TLR signaling, type I IFN signaling, and expression of TNFR1. On the other hand, expression of EPOR was associated with key immune-regulatory genes, such as Apoe and Tnfaip3. These results suggested that the effects of EPOR were similar between splenic and migratory cDC1s.
Given that disruption of EPOR signaling was shown to prevent tolerogenic maturation and support immunogenic priming, Zhang et al. investigated whether this might provide a benefit in the context of cancer immunotherapy. In tumor models, the researchers found that EPOR was preferentially expressed on mature CCR7+ regulatory cDC1s with increased expression of CD40, CD80, CD86, MHC-I and PD-L1. In tdLNs, a similar effect was observed on migratory cDC1s. Further, serum EPO positively correlated with tumor growth, likely due to its effect on EPOR+ cDC1s. When EPOR was deleted in cDC1s, tumor-bearing mice showed increased CD80/86+ tumor cDC1s, CD40 expression on migratory cDC1s in tdLNs, tumor antigen-specific CD8+ T cell priming in tdLNs, Tpex cell proliferation, tumor infiltration, CD8+ T cells among among TILs, and expression of effector molecules, as well as decreased T cell exhaustion and tumor growth. Among CD4+ T cells, conventional CD4+ T cells increased, while Tregs decreased. Loss of EPOR also synergized with anti-PD-1 in B16F10-OVA tumor models.
Overall, these results suggest that EPOR signaling induces a tolerogenic phenotype in cDC1s. In tumor models, deletion of EPOR signaling in cDC1s limited Treg priming, promoted antitumour CD4+ and CD8+ T cell immunity, restrained tumor growth, and enhanced the efficacy of immune checkpoint blockade. In addition to providing key insights into the maturation of tolerogenic cDC1s, these results point to potential opportunities to target EPOR to enhance cancer immunotherapy.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Xiangyue Zhang and lead author Edgar G. Engleman answered our questions.

What was the most surprising finding of this study for you?
cDC1s are central to the cancer immunity cycle and antiviral immunity due to their superior cross-presentation capacity; meanwhile, they are also capable of inducing antigen-specific regulatory T cells, a function that has remained poorly defined and underappreciated in the context of cross-presentation. To address this gap, we investigated how cDC1s acquire a tolerogenic maturation state using the rigorous TLI/ATS tolerance model. Transcriptomic profiling of cDC1s unexpectedly revealed strong induction of the erythropoietin receptor (EPOR). Defining this pathway required substantial technical innovation and sustained conceptual judgment in the absence of precedent. Genetic analyses ultimately identified EPOR as a key regulator of cDC1 tolerogenic programming, fundamentally reshaping our understanding of how cDC1 maturation governs adaptive T cell immunity.
What is the outlook?
This study identifies EPOR as a master regulator of tolerogenic maturation in cDC1s, redefining a conserved mechanism that governs the balance between tolerogenic and immunogenic function. EPOR signaling programs cDC1s to impose durable tolerance in both CD4⁺ and CD8⁺ T cells toward cell-associated antigens, overturning a long-standing paradigm in cDC1 immunobiology. Importantly, the EPO–EPOR axis represents an immediately actionable therapeutic lever: EPOR agonism offers a rational strategy to induce immune tolerance in transplantation and autoimmunity, whereas EPOR antagonism provides a new avenue to disrupt pathological tolerance and enhance antitumor immunity. Together, these findings position EPOR-driven cDC1 programming as a conceptual and translational inflection point for the field.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
XZ: Maintain humility and openness, and seek opportunities for deeper engagement with outstanding immunologists and their works.
Who or what has been a major source of inspiration or motivation for you throughout your career?
XZ: I am deeply grateful to my current mentor, Professor Edgar G. Engleman, and to my Ph.D. supervisor, Professor Reinhold Förster. Reinhold instilled in me the guiding principle that, when faced with uncertainty or frustration, one should return to the data, trusting that it will illuminate the next steps. As a world-renowned translational immunologist, Ed has profoundly shaped my scientific perspective, teaching me that immunological discovery should not only advance fundamental understanding, but also carry meaningful translational relevance and potential benefit for patients. Throughout my career, I have been driven by a deep passion for immunology – particularly immune tolerance –and a commitment to pursuing clear, principled answers to complex biological questions.




