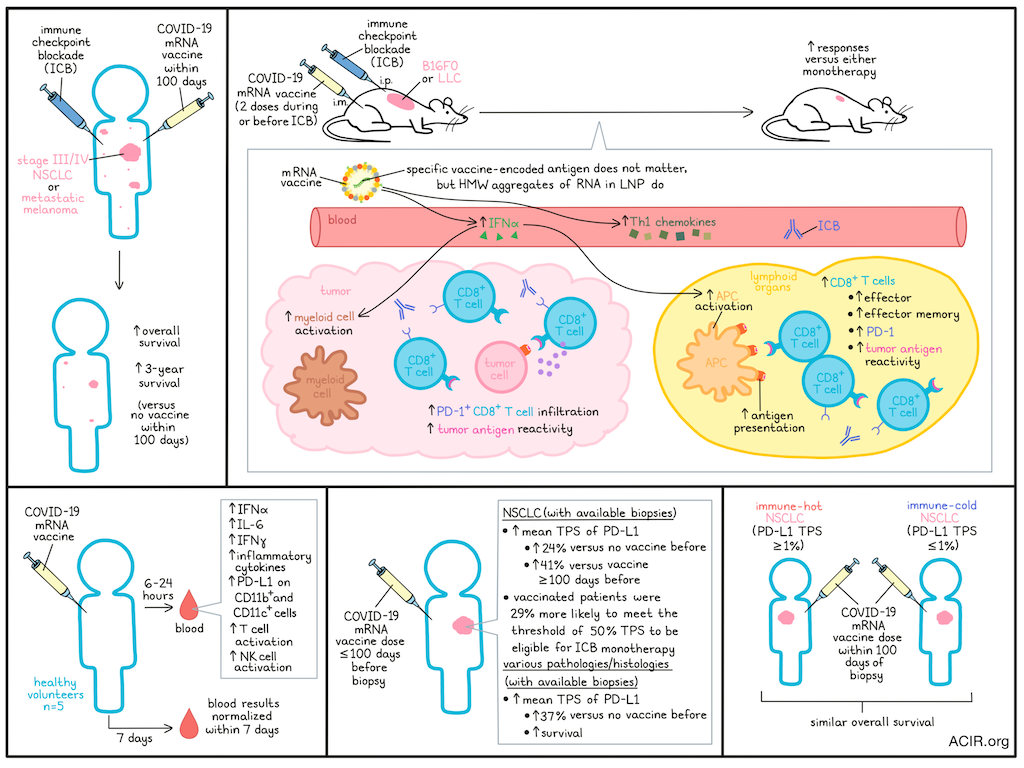
Improving the efficacy of immune checkpoint blockade (ICB) for a larger group of patients, particularly those with immune-cold tumors, is one of the major goals of current immunotherapy research. Based on case reports of patients who had spontaneous tumor regression after receiving COVID-19 mRNA vaccines, Grippin, Marconi, Copling, Li, et al. investigated the impact of these vaccines in patients undergoing ICB treatment. Their results were recently published in Nature.
The researchers first compared a cohort of patients treated with ICB for stage III/IV non-small cell lung cancer (NSCLC) who received a COVID-19 mRNA vaccine within 100 days of ICB initiation (n=180) to those who did not receive a vaccine (n=704) in this period. In this cohort, those who had received the vaccine had significantly improved median overall survival (OS) and 3-year survival. This survival benefit was observed in both stage III unresectable and stage IV NSCLC. The vaccine manufacturer, whether it was the first dose, or whether patients received more than one dose did not impact the survival benefit observed. In patients who received an mRNA vaccine followed by chemotherapy instead of ICB, or who received a flu or a pneumonia vaccine followed by ICB, survival was not improved. Similar results were found in a cohort of patients with metastatic melanoma.
To study these effects in more detail, the researchers synthesized the mRNA construct of the BNT162b2 vaccine and encapsulated it in lipid nanoparticles (LNPs) to be used in animal models. The B16F10 melanoma and Lewis lung carcinoma (LLC) murine models were used for experiments, as they respond poorly to ICB treatment. Mice were treated with two vaccine doses in conjunction with ICB. This combination was more effective than either monotherapy in mice with established B16F0 tumors, subcutaneous (s.c) LLC, established s.c. LLC, or orthotopic LLC. Similar effects were found when the vaccine was provided before ICB initiation.
To determine the mechanistic effects of this treatment combination, mice with B16F0 tumors were treated with the RNA-LNPs and/or anti-PD-L1 antibodies. This combination treatment reduced tumor growth. Blockade of IFNAR1 (to block type I IFN signaling) removed this antitumor response, and administration of type I IFN could bring it back, suggesting IFN plays an important mechanistic role. Similar results were obtained when the mRNA targeting spike was replaced with mRNA encoding a cytomegalovirus antigen, which is not a relevant antigen for B16F0, suggesting the antigen is not of importance for these effects. Further structural studies showed that replacing N1-methyl-pseudouridine with uridine enhanced the effect for some RNAs, but not others; reducing the levels of observable double-stranded RNA (dsRNA) did not alter activity; and the formulation of RNA in the LNPs created high molecular weight (HMW) aggregates with features resembling those of dsRNA, which may be responsible for activating the MDA-5 pathway.
The researchers found that levels of IFNα and Th1 chemokines were significantly higher in the RNA-LNP monotherapy and the combination treatment groups. This increase correlated with activation of APCs, including DCs, macrophages, and Ly6C+MHC-II+ cells in lymphoid organs, which could be abrogated by IFNAR1 blockade. Myeloid activation was also observed in the tumor, with IFNAR1-dependent increases in activated Ly6C+ cells. To determine if this APC activation resulted in increased tumor antigen presentation, treatment was assessed in mice with B16F10-OVA tumors. RNA-LNP treatment resulted in increased ovalbumin antigen presentation by APCs in the lymphoid organs, particularly Ly6C+ cells, in the presence of costimulatory molecules.
Besides innate immune activation, CD8+ T cells expanded, including effector and effector memory populations, which all had increased PD-1 expression. In the spleen, combination treatment resulted in an expansion of tetramer-reactive T cells for six melanoma-associated antigens, and these cells were tumor-reactive in vitro. PD-1+CD8+ T cell infiltration increased in B16F0 tumors after combination treatment, making them the most abundant population within the CD3+ T cell compartment. These tumoral CD8+ T cells were twice as likely to be tetramer-reactive than those from control mice.
To determine whether similar immune activation occurred in humans, blood samples from five healthy volunteers were assessed before and after receipt of a COVID-19 mRNA vaccine. IFNα was the most upregulated cytokine, and increased approximately 280-fold compared to baseline. Further, IL-6 and IFNγ were significantly elevated after 6 hours, and many inflammatory cytokines increased at 24-hours post-vaccination. Immunization increased PD-L1 expression on CD11b+ and CD11c+ cells, and increased NK cell and T cell activation. The cytokine responses and cellular phenotypes normalized by 7 days post-vaccine.
The researchers then assessed the impact of immune activation on tumoral PD-L1 expression. In a cohort comprising patients with NSCLC for whom biopsies reporting the tumor proportion score (TPS) of PD-L1 were available, those who had received the COVID-19 mRNA vaccine <100 days before biopsy had a 24% increase in mean TPS compared to those who had received no vaccines before biopsy, and a 41% increase relative to patients who received the vaccine ≥100 days before biopsy. A TPS of 50% is clinically important, as it determines eligibility for ICB monotherapy, and patients who received the vaccine were 29% more likely to meet this threshold than unvaccinated patients. In contrast, and consistent with the prior survival data, pre-biopsy influenza and pneumonia vaccines were not associated with TPS changes.
In a cohort of patients with various pathologies and histologies, receipt of a COVID-19 mRNA vaccine within 100 days before the biopsy was associated with a 37% increase in TPS, and the patients in this group who received ICB within 100 days of vaccination had significantly improved survival compared to unvaccinated patients.
To determine whether the vaccine could restore immune sensitivity in patients with immune-cold tumors, pre-vaccine TPS <1% in NSCLC was used as a surrogate for this type of tumor, as it lacks ICB efficacy. Among stage IV NSCLC with baseline <1% TPS, those who received a COVID-19 mRNA vaccine within 100 days of ICB initiation had a similar OS to that of patients with >1% TPS, suggesting the vaccine restores sensitivity to ICB.
Overall, these data suggest that mRNA vaccines targeting non-tumoral antigens can stimulate antitumor immunity and sensitize tumors to ICB treatment. Therefore, the timing of mRNA vaccines relative to ICB treatment might be an important consideration for the management of patients with various cancer types. Further, these data could inform studies into universal mRNA therapies aimed at sensitizing tumors to immunotherapy.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, co-first author Adam Grippin answered our questions.

What was the most surprising finding of this study for you?
This study was full of exciting findings, but the most exciting for me was how consistently effective these vaccines have been in so many treatment settings. First, we found a truly remarkable signal in our retrospective clinical data that suggested we were on to something big. In subsequent experiments, these vaccines produced consistently impressive effects in every preclinical model they were tested in, including some very difficult-to-treat established and orthotopic tumor models. When we replicated these findings at multiple institutions, we knew we had a story to tell.
What is the outlook?
Although we think our findings are incredibly exciting and thought-provoking, this work is preliminary and needs to be validated before we make any changes to how we manage patients in the clinic. As a result, the most important next step is to test whether widely available COVID mRNA vaccines can be repurposed as tools to sensitize tumors to immune checkpoint blockade. We are currently designing a randomized phase III clinical trial to do just that.
The next most important step is to build on the key finding that mRNA vaccines targeting non-tumor antigens can be used as universal immune stimulants to sensitize tumors to immunotherapy. Although we have demonstrated impressive effects with COVID mRNA vaccines, there is no reason to think that these are the best possible vaccines for this purpose. We are excited to lead a new wave of universal RNA therapeutics that we hope will sensitize tumors to immunotherapy even more strongly.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
My advice to my younger self would be to go all-in on the science that you truly believe can change the world. It’s easy to get distracted by low-hanging fruit or side projects that seem immediately rewarding, but every extra commitment takes time away from your central mission. I’ve been fortunate to have incredible mentors who encouraged me to stay focused on work that matters most, and I’ve loved every moment spent with my team of motivated people united by the belief that we are building something transformative.




