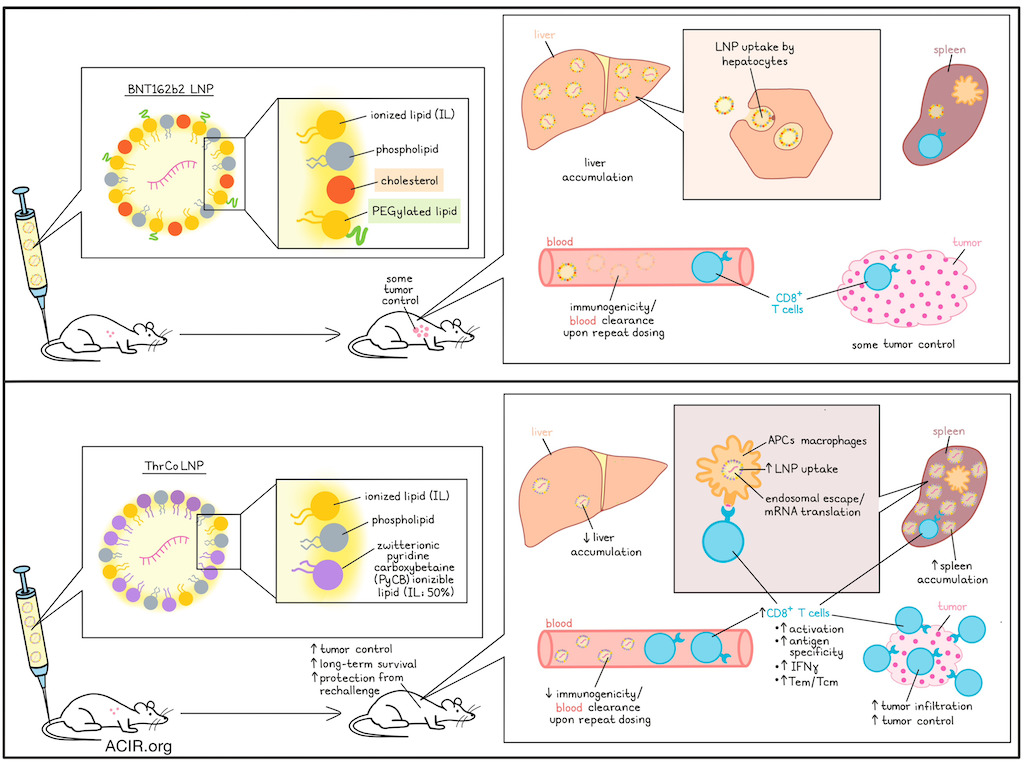
The spleen has gained interest as a vaccination target, as high uptake of therapeutic mRNA cancer vaccines by antigen-presenting cells (APCs) is needed for therapeutic efficacy. However, current formulations of mRNA vaccines contain lipid nanoparticles (LNPs) that accumulate in the liver. Further, polyethylene glycol-modified (PEGylated) lipids on LNPs can induce PEG immunogenicity with repeated dosing. To improve the efficacy of therapeutic LNP vaccines, Zhao, Tian, Wang, et al. described their development of a new vaccine formulation to overcome these challenges in a recent publication in Science Advances.
The researchers synthesized zwitterionic pyridine carboxybetaine ionizable lipids (PyCB ILs) as a substitute for the cholesterol and PEGylated lipids present in the LNPs of the BNT162b2 mRNA vaccine to assess whether this change could help target the spleen. They hypothesized that reducing cholesterol would decrease the lipoprotein binding capacity and efficient hepatocyte entry, resulting in less accumulation in the liver, and that reducing PEGylated lipids would prevent immunogenicity upon repeated dosing.
The researchers formulated a series of LNPs with increasing percentages of PyCB ILs. The RNA and the LNPs were fluorescently labeled and then tracked for distribution in C57BL/6 mice using in vivo imaging after intravenous (i.v.) injection. Complete removal of cholesterol and PEGylated lipids significantly reduced LNP accumulation and mRNA translation in the liver, but minimally increased spleen accumulation. At a percentage of 45, more LNPs were in the spleen and fewer were in the liver for the PyCB IL formulation than the BNT162b2 LNPs. This resulted in higher spleen-specific mRNA translation. The optimal percentage of PyCB IL was determined at 50%, and this formulation (ThrCo LNP) was used in subsequent experiments.
In vivo imaging experiments showed that compared to the BNT162b2 LNPs, liver accumulation of dye-labeled ThrCo LNPs was reduced, with a 2.6-fold increase in the spleen, accompanied by 4.5-fold higher expression of luciferase mRNA in the spleen. Toxicity and stability experiments showed similar or better results for ThrCo LNPs compared with BNT162b LNPs.
The cellular uptake mechanisms of ThrCo LNPs were assessed in vitro using various pathway-specific inhibitors, which showed that it was primarily internalized via endocytosis. Further assessment showed efficient endosomal escape, effective membrane disruption capability with decreasing pH, allowing the LNPs to dissociate, resulting in mRNA release. Further, no red blood cell lysis was observed following incubation under physiological conditions, suggesting it is biocompatible in the blood.
To determine whether the vaccine could efficiently deliver mRNA to APCs, splenic immune cells were assessed in genetically engineered Ai14 mice, which stably express tdTomato following intracellular delivery of Cre recombinase mRNA, allowing identification of cell types taking up and translating the encapsulated Cre mRNA. I.v. injection of ThrCo LNPs containing Cre mRNA induced significantly higher proportions of tomato-expressing dendritic cells and macrophages than BNT162b2 LNPs containing Cre mRNA.
To assess induction of specific CD8+ T cell responses, mice were vaccinated with different LNP formulations containing mRNA encoding OVA as the vaccine target antigen (a priming dose followed by booster doses on day 7 and 14), and T cells were assessed on day 17. The ThrCo LNP-based vaccine induced higher CD8+ T cell activation levels than the BNT162b2 LNP-based formulation (9.06% vs. 3.96%).
Next, the researchers investigated vaccine efficacy after repeated administrations, as this might be required for cancer therapy. LNP mRNA delivery efficiencies were assessed under two injection conditions. Under the first condition, two injections were given with EGFP mRNA and a third injection was given with Luc mRNA. Under the second condition, only a single injection of Luc mRNA was given to establish whether PEG immunogenicity reduces the delivery efficiency of the Luc mRNA. While two prior EGFP mRNA injections of the BNT162b2 formulation resulted in reduced Luc expression, there was no decline when ThrCo LNPs were used. This was consistent with measurement of a lower protein adsorption with ThrCo LNPs, which reduces antibody recognition and binding, likely due to the zwitterionic groups in the formulation.
Moving to efficacy assessment in a tumor model, C57BL/6 mice were inoculated i.v. with OVA-expressing B16F10 cells, and vaccinated with the two LNP formulations encapsulating OVA mRNA on days 1, 6, and 11. On day 21, the presence of metastases in the lungs was assessed. The BNT162b2 LNP formulation resulted in fewer tumor nodules than controls, but in mice vaccinated with the ThrCo LNP formulation, no nodules were detected. Mice receiving the ThrCo LNP formulation had more OVA-specific CD8+ T cells in the spleen and blood, more IFNγ expression on those cells, and increased proportions of effector memory (Tem) and central memory (Tcm) T cells, compared to the mice injected with the BNT162b2 formulation.
To assess the ability to prevent tumor recurrence, mice were injected i.v. with B16F10-OVA cells, followed by vaccination, and on day 21, mice received subcutaneous injection of B16F10-OVA in one flank and EO771 mammary tumor cells in the other flank. The recurrence of the B16F10 tumors was suppressed entirely in 4/5 mice vaccinated with the ThrCo LNP formulation, with only slight suppression of the E0771 tumor. Within the tumors, CD8+ T cells and IFNγ levels increased. To assess survival, B16F10-OVA tumor-bearing mice were vaccinated i.v. 6 days after tumor implantation and followed for 50 days. The ThrCo LNP-based vaccines suppressed tumor growth and improved survival from 0% to 60% compared to the BNT162b2 LNP formulation. These mice also had higher levels of OVA-specific CD8+ T cells in the spleen and increased IFNγ expression. On day 50 in surviving animals, the ThrCo LNP treatment group had higher tumor infiltration of CD8+ T cells, OVA-specific CD8+ T cell infiltration, and Tem cells than mice treated with control or the BNT162b2-based formulation.
Overall, these data suggest that altering the formulation of LNPs for cancer mRNA vaccines results in improved splenic delivery of mRNA, which enhances antigen-specific CD8+ T cell responses and improves tumor control in murine models. Therefore, this formulation may help overcome some of the current challenges with the therapeutic efficacy of cancer mRNA vaccine strategies.
Write-up by Maartje Wouters, image by Lauren Hitchings




