Cytotoxic cancer treatments, like chemotherapy and radiation, have the potential to synergize with immunotherapies through direct killing of tumor cells, which leads to the release of antigens and damage-associated molecular patterns. However, this potential synergy is often hindered when indiscriminate cytotoxicity kills immune cells as well. To more selectively target cancer cells, Gujar and Cui et al. developed N17350 – a therapeutic elastase that induces immunogenic cell death in cancer cells via the ‘‘neutrophil elastase pathway’’, and is optimized for intratumoral delivery. In work recently published in Cell Reports Medicine, N17350 was tested in a wide range of settings to evaluate its antitumor efficacy and potential for clinical use.
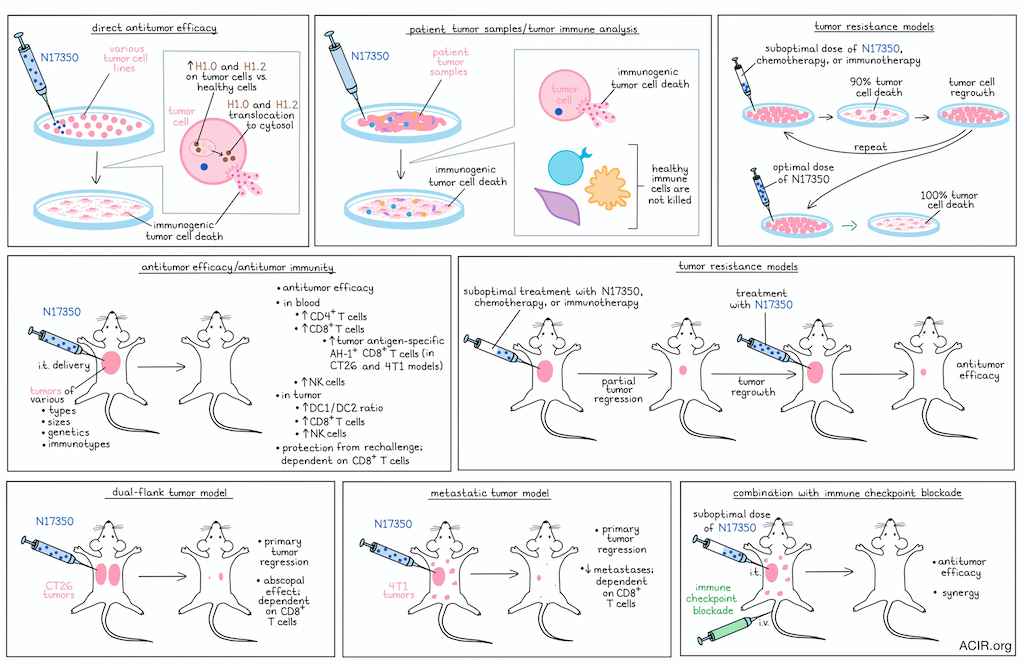
To begin, the researchers tested N17350 in vitro against a broad range of cancer cell lines, and found that it effectively killed lung, breast, colon, ovarian, melanoma, and other cancer types with similar efficacy. N17350 was also effective against lung cancer cells with distinct KRAS mutations and against primary human tumor cells isolated from primary high-grade serous ovarian cancers, which lack specific driver mutations.
Evaluating N17350 in vivo, the researchers tested it against 12 diverse syngeneic and xenograft models of lung, colon, breast, prostate, and esophageal tumors in mice. Across these models, N17350 induced tumor regression against tumors of varying sizes, genetics, and immunotypes, outperforming carboplatin chemotherapy or KRAS-targeted therapies (in relevant models).
Having established the tumor-killing capacity of N17350, the researchers investigated whether N17350 also killed immune cells. In samples from patients with ovarian cancer, N17350 selectively killed cancer cells, without killing CD45+ immune cells or fibroblasts from the same patients’ tumor, omental tissue, intraperitoneal fluid, or blood, exhibiting a wide therapeutic window (>100X). Similar selective cancer killing was observed across other cancer and non-cancer cell lines.
Digging deeper, the researchers found that N17350 increased markers of immunogenic cell death (ICD) across samples from patients with ovarian cancer, consistent with previous research into the mechanism of action of the neutrophil-derived elastase ELANE. Hypothesizing that this ICD could enhance antitumor immune responses, the researchers found that in both immunologically “hot” and “cold” tumor models, treatment with a single intratumoral dose of N17350 rapidly induced tumor regression and increased levels of CD8+ T cells, CD4+ T cells, and natural killer (NK) cells in the blood. Twelve days after treatment, the DC1/DC2 ratio and levels of NK cells and CD8+ T cells in the tumor were increased, as were systemic immune responses, including an increase in tumor-antigen-specific AH-1+ CD8+ T cells in blood in relevant models, which were enriched in memory precursor and effector subsets. In the same models, chemotherapies slowed tumor growth, but had minimal or negative effects on the immune system.
Next, Gujar and Cui et al. evaluated whether their observations related to antitumor immunity translated to distant tumor control. To this end, they evaluated abscopal effects in a dual-flank CT26 tumor model, and found that N17350 induced tumor regression in both injected and non-injected tumors. This effect could not be attributed to “spillover” of N17350 from the primary tumor, and was instead dependent on CD8+ T cells. Similarly, in a 4T1 spontaneous lung metastasis model that is typically considered immune-cold, injection of primary tumors with N17350 induced regression of both primary tumors and metastases, dependent on CD8+ T cell Finally, the researchers rechallenged mice 90 or 150 days after initial NY17350 curative treatment and showed that the mice were protected, again dependent on CD8+ T cells. While systemic antitumor could also be induced by injecting CT26 cells killed with oxaliplatin into naive mice, it could not be induced by direct treatment of tumors with oxaliplatin, likely due to its toxicity to immune cells.
To determine whether N17350 could be used to enhance the efficacy of immune checkpoint blockade (ICB), the researchers used the murine 4T1 tumor model, where anti-CTLA-4 alone was ineffective. Here, a single dose of N17350 reduced the growth of both primary tumors and metastases and enhanced the efficacy of anti-CTLA-4, leading to improved tumor control and survival. Similar results were observed in other tumor models and in combination with anti-PD-1. For comparison, oxaliplatin did not show the same synergistic effects with ICB.
Another challenge associated with many cytotoxic cancer treatments is the development of resistance with repeat dosing. Evaluating possible resistance to N17350, the researchers treated cancer cells in vitro with doses sufficient to induce 90% killing, and allowed for cells to regrow between doses. After 5 cycles of repeat dosing, cells remained fully susceptible to N17350. Similar effects were observed in vivo across five tumor models in Nude mice, where N17350 retained cytotoxicity against tumors that were not fully cleared after earlier suboptimal doses. Further, N17350 was effective against tumors that had developed resistance to other chemotherapies, as well as in a tumor model of anti-PD-1 resistance, suggesting that it could overcome cross-resistance, and could be effective in patients with extensive treatment histories.
Finally, the researchers explored the suspected mechanism of action of N17350, and showed that, in line with previous research, it induced ELANE-mediated hallmarks in multiple cancer cell lines. Expression profiling revealed that cancer cells expressed significantly higher H1.0 and H1.2 proteins compared to non-cancer cells, and that in cancer cells, these proteins showed rapid cytosolic translocation after treatment with N17350. CRISPR/Cas9-mediated knockdown of H1.0 or H1.2 proteins in cancer cells reduced the antitumor efficacy N17350. Further, in TCGA and TIMER data from patients, histone H1 isoforms were overexpressed in solid tumors compared to adjacent tissues. Elevated H1.0 and H1.2 protein levels in tumor versus non-tumor or immune cells were also observed via immunostaining of tumor microarrays from a variety of cancer types, and in analyses of ovarian cancer samples.
Altogether, Gujar and Cui et al. evaluated N17350 across 30 cancer cell lines, 15 tumor models, and 45 patient samples. Their results provide extensive preclinical evidence that N17350 treatment selectively induced immunogenic cell death in cancer cells based on their increased expression of linker histone H1.0 and H1.2 proteins, even in settings of repeat dosing or cross-resistance to other therapies. Importantly, N17350 spared critical immune cells, allowing for N17350-induced ICD to support antitumor immunity and durable immune memory. These findings support the advancement of N17350 to first-in-human clinical trials.
Write-up and image by Lauren Hitchings




