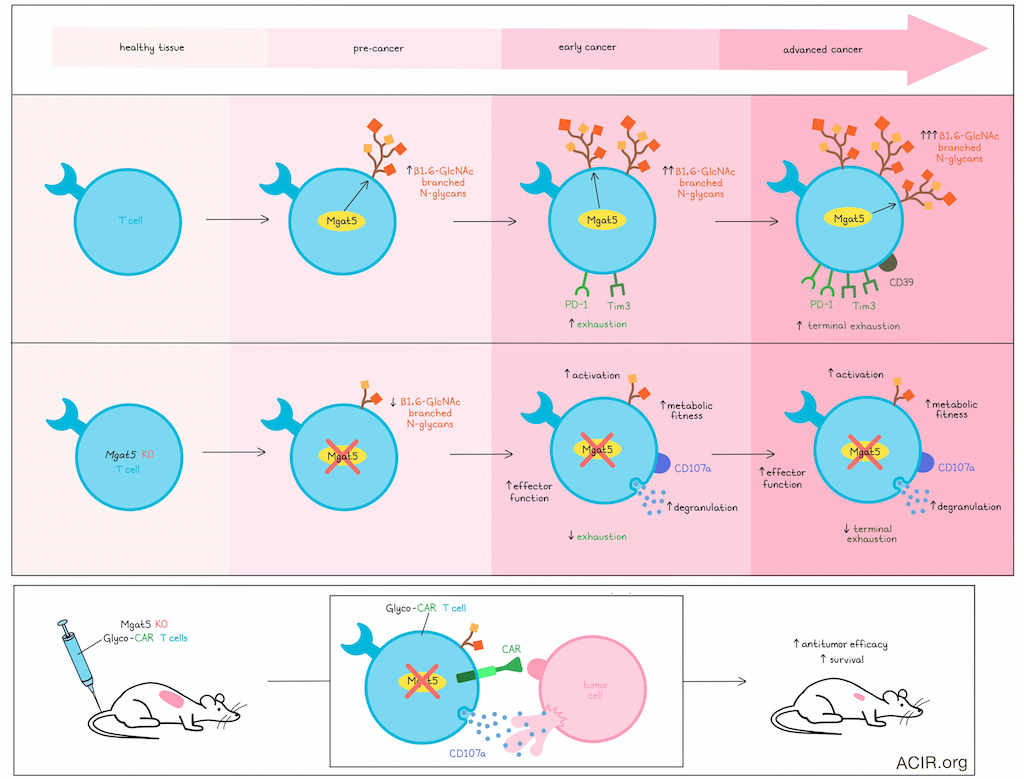
Changes in glycosylation are a hallmark of cancer, and recent work has suggested that branched N-glycans on T cells may play a role in limiting their antitumor activity. In recent work, Azevedo et al. investigated the T cell glycome within tumors to better understand whether and how expression of aberrant glycans on T cells affects antitumor functions over the course of tumor development. Their results were recently published in Cancer Immunology Research.
To characterize the dynamics of N-gylcosylation in tumor-infiltrating lymphocytes, Azevedo et al. selected serrated colorectal cancer (Ser CRC) as a primary model, due to its high mutation burden and strong immune infiltration. In analyses of Ser CRC samples from patients, the researchers observed increased expression of stromal β1,6-GlcNAc branched N-glycans (by L-PHA staining) starting in pre-malignant lesions and increasing with more advanced disease. This increase also tracked with increased infiltration of T cells and CD8+ T cells, loss of granzyme B, and increased expression of MGAT5 (encoding a glycosyltransferase involved in the formation of β1,6-GlcNAc branched N-glycans). Similar results were observed based on flow cytometry of fresh colonic biopsies from patients with Ser CRC, which showed increased CD8+ T cells and a trend towards increased β1,6-GlcNAc branched N-glycans on CD8+ T cells. In pre-malignant lesions, β1,6-GlcNAc branched N-glycans negatively correlated with IFNγ and granzyme B production. Another cohort of Ser CRC patient data showed a positive correlation between MGAT5 expression and expression of CTLA-4 and TIGIT. Similarly, a cohort of patients with Lynch Syndrome showed increases in T cells and MGAT5 expression with disease progression, and MGAT5 expression correlated with increased PD-1, CTLA-4, LAG3, and TIGIT.
Next, the researchers tested whether N-glycosylation might affect T cell effector functions or exhaustion. In an MC38-OVA tumor-bearing mouse model treated with OT-I cells, the researchers observed an emergence of exhausted (PD-1+Tim3+) and terminally exhausted (PD-1+Tim3+CD39+) T cells that coincided with increasing levels of β1,6-GlcNAc branched N-glycans on CD8+ T cells. In vitro modeling of acute and chronic stimulation under conditions of normoxia or hypoxia showed that chronic activation under hypoxia (mimicking a hostile TME) induced the highest levels of PD1+Tim3+ exhausted T cells and the highest expression of β1,6-GlcNAc branched N-glycans.
To further explore the relationship between β1,6-GlcNAc branched N-glycans and exhaustion, Azevedo et al. used CRISPR/Cas9 to knock out each of 3 glycosyltransferases – Mgat1, Mgat2, and Mgat5, involved in different stages in the formation of β1,6-GlcNAc branched N-glycans – in OT-I cells. Knockout of Mgat5 most prominently decreased β1,6- GlcNAc branched N-glycans and PD-1+Tim3+ exhausted cells following activation. Expression of Tcf1 (precursor exhaustion) and Tox (late exhaustion) were also reduced, with fewer cells exhibiting a Tbet+Eomes+ exhausted phenotype. Similar results were observed under conditions of chronic stimulation and hypoxia, supporting an early role for Mgat5 and β1,6-GlcNAc branched N-glycans in inducing T cell exhaustion.
Digging deeper, the research assessed the impact of β1,6-GlcNAc branched N-glycans on T cell fitness and metabolism and found that Mgat5 KO CD8+ T cells showed increased basal and maximal respiratory capacity, increased glycolysis and glycolytic capacity, and increased CD69 expression (indicative of activation). In line with this, naive T cells isolated from Mgat5 KO mice showed increased CD69, proliferation, TCR signaling, and production of IFNγ and TNFα. Similar results were observed in mice with Mgat1 or Mgat2 knocked out in T and B cells, but to a lesser extent.
Next, Azevedo et al. explored whether Mgat5 KO CD8+ T cells might show enhanced antitumor activity. Indeed, when co-cultured with MC38-OVA cells, these cells again showed enhanced activation and reduced exhaustion, along with enhanced tumor cell killing and cytolysis, including increased degranulation (CD107a). Similar results were observed in T cells from Rag2-/- OT-I Mgat5-/- mice, which showed enhanced tumor cell killing relative to those from Rag2-/- OT-I WT mice. Additionally, overexpression of Mgat5 in CD8+ OT-I T cells resulted in increased branched N-glycans and reduced tumor cell killing, further establishing the immunoregulatory role of Mgat5.
To test the potential of Mgat5 deletion for improving antitumor efficacy in vivo, the researchers transferred Mgat5 KO OT-I cells into MC38-OVA-bearing mice and found that these cells significantly suppressed tumor growth and improved overall survival compared to transfer of WT OT-I cells. Similarly, MC38 tumor growth was reduced in Rag2-/- OT-I Mgat5-/- mice versus Rag2-/- OT-I WT mice, with a trend towards longer survival. Isolated Mgat5 KO OT-I T cells isolated from tumors exhibited higher cytotoxic capacity upon ex vivo restimulation. These also showed a trend towards decreased exhaustion (PD-1+Tim3+ CD8+ T cells) and a significant decrease in terminal exhaustion (CD39+ CD8+ T cells). RNAseq further revealed an increase in expression of genes related to survival, effector functions, and cellular respiration/oxidative phosphorylation, and a decrease in expression of genes related to stemness and memory in T cells.
Moving towards a more clinically relevant model, Azevedo et al. isolated CD3+ T cells from human PBMCs, transduced them with CD19-CARs, and used CRISPR/Cas9 to knock out MGAT5. In coculture with A549 human lung cancer cells expressing CD19, these Glyco-CAR T cells showed increased degranulation (CD107a expression) in both CD4+ and CD8+ populations, and demonstrated enhanced tumor cell killing compared to standard CAR T cells. In NSG mice bearing established A549-hCD19 tumors, treatment with Glyco-CAR T cells induced tumor growth inhibition and prolonged survived over control CAR T cells.
Together, these results show that hostile TMEs drive aberrant glycosylation of T cells, mediated in part by Mgat5 expression, driving them towards exhaustion starting in early premalignant lesions. Deletion of Mgat5 in T cells reduced the presence of β1,6-GlcNAc branched N-glycans, enhanced their metabolic fitness, and increased T cell activation and effector functions, including degranulation and cytolytic antitumor activity. When applied to human CAR T cells, MGAT5 knockout enhanced their in vitro and in vivo efficacy against solid tumors, which could translate to enhanced clinical efficacy in the future.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, lead author Salomé Pinho answered our questions.

What was the most surprising finding of this study for you?
T cell therapies such as CAR T cells are not yet effective in solid tumors, due to the complexity of tumor microenvironment. We here reveal that branched N-glycosylation emerges at the surface of infiltrating T cells in pre-malignancies, imposing an exhausted and dysfunctional phenotype. We targeted Mgat5-mediated branched N-glycans on CD8+ (CAR-) T cells as a strategy to prevent exhaustion, enhancing cytotoxicity and antitumor T cell activity.
We here revealed the power of glyco-engineering CD8+ T cells in boosting the efficacy of cellular immunotherapy. We demonstrated that by removing a specific glycogene, MGAT5, the surface glycocalyx of T cells is efficiently reprogrammed, preventing T cell exhaustion and increasing cytotoxicity and tumor killing.
What is the outlook?
These findings illuminate T cell glycoengineering as an innovative strategy to unlock T cell effectiveness in cancer immunotherapy, laying foundation for proposing glycoengineering of CAR T cells as an effective strategy to create a new generation of super Glyco-CAR T cells with superior efficacy in solid tumors.
Who or what has been a major source of inspiration or motivation for you throughout your career?
A major source of inspiration throughout my career has been the transformative power of science itself — not just as a tool to improve health, but as a driver of societal progress and economic development. What continues to motivate me is the understanding that scientific discoveries only reach their full potential when pursued collaboratively, across disciplines and across borders. Witnessing how research can lead to innovative therapies that save millions of lives, new technologies, and innovations that can transform society and the economy has been reinforcing my strong belief in the power of science as a collective endeavor. This sense of purpose — working together to turn knowledge into tangible benefits — has been my guiding motivation.




