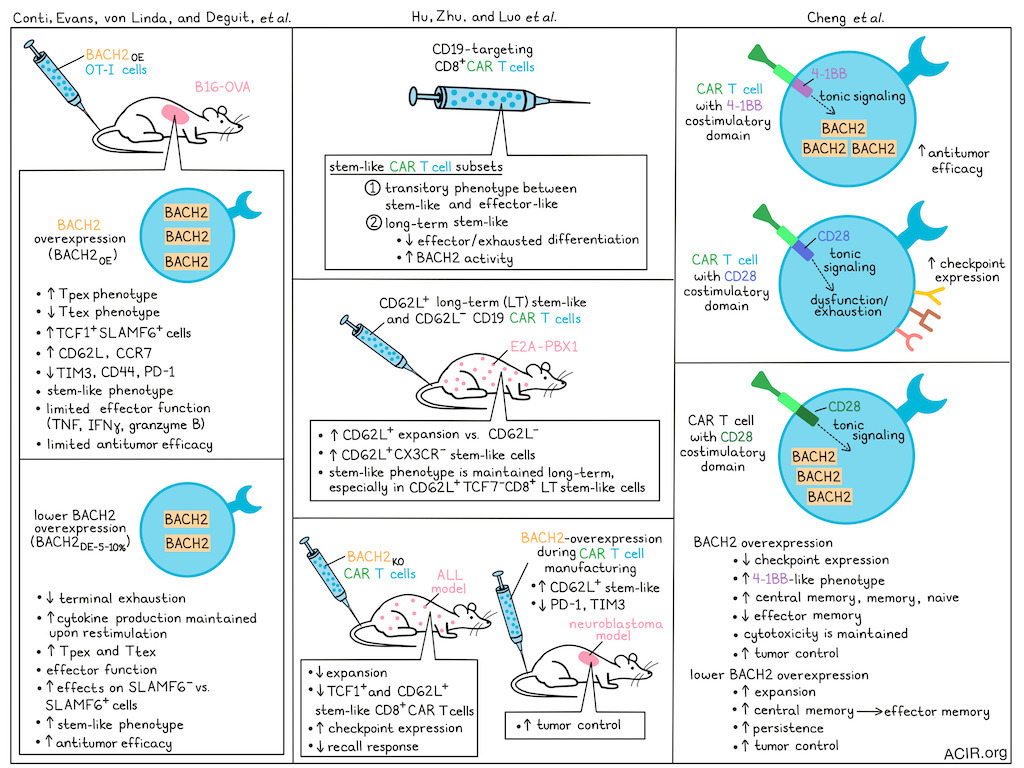
Adoptive T cell transfer (ACT) and CAR-T treatment strategies are limited in their efficacy against solid tumors, as chronic antigen exposure induces exhaustion phenotypes, with reduced proliferation and effector function, limited persistence, and reduced therapeutic efficacy as consequences. Three recent back-to-back publications in Nature Immunology assessed the role of BACH2 in these processes. Conti, Evans, von Linde, Deguit, et al. assessed whether BACH2 overexpression can improve ACT efficacy; Hu, Zhu, Luo, et al. investigated the role of BACH2 in maintenance of stem-Like CAR-T cells; and Chang et al. determined the role of BACH2 in tonic signaling and how it can impact CAR-T lineage and function.
BACH2 was known to maintain the stem cell-like memory cell pool through restriction of terminal differentiation during acute and chronic infections in mice. Looking for parallels in human cancers, Conti, Evans, von Linde, Deguit, et al. used transcriptional analysis to show that BACH2 mRNA expression in intratumoral T cells was highest in the naive and memory populations, and correlated with TCF7 and IL7R expression. BACH2 expression decreased with T cell differentiation stages, with effector cells having intermediate expression and terminal effector memory (Tem) cells having the lowest expression levels.
To assess whether BACH2 overexpression could improve the antitumor efficacy of ACT, the researchers retrovirally transduced OT-I cells to overexpress BACH2 (BACH2OE) for ACT into B16-OVA tumor-bearing mice. In tumors, the BACHOE-transduced cells clustered as a population with a progenitor-exhausted (Tpex) phenotype. Further, the proportion of TCF1+Slamf6+ cells and expression of CD62L (Sell) and CCR7 increased, while TIM3, CD44, and PD-1 expression decreased. While these cells maintained a stem-like phenotype, they exhibited limited effector function: they produced very little TNF, IFNγ, and granzyme B upon ex vivo restimulation, and had impaired antitumor responses.
To determine whether fine-tuning BACH2 expression levels leads to a stem-like phenotype that maintains effector function, the researchers then used mutated translational readthrough motifs to fine-tune BACH2 expression and achieved low-dose expression at 10% (BACH2DE-10%) or 5% (BACH2DE-5%) of the BACH2OE level. Both doses generated intermediate expression levels similar to those observed in endogenous central memory T cells (Tcm). Following chronic stimulation, there was a reduction in terminally exhausted T cells (Ttex), and the cells maintained cytokine production, suggesting sustained effector function.
To test the impact on ACT efficacy, the B16-OVA tumors were treated with native or expression-modified OT-1 T cells. BACH2OE did not improve antitumor efficacy, while both BACH2DE vectors improved the response. BACH2DE resulted in increased levels of Tpex and Ttex cells, while BACH2OE resulted in loss of Ttex cells. Ex vivo experiments showed that BACH2DE produced effector molecules, and tumors of treated mice contained more cytokine-producing cells. Specifically, BACH2DE impacted Slamf6- cells more than Slamf6+ cells , resulting in acquisition of stem-like transcriptional characteristics.
In the second paper, Hu, Zhu, Luo, et al. transcriptionally clustered murine CD8+ CD19-targeting CAR-T and identified a stem-like subset that could be clustered into two subgroups: one displaying a transitory state between stem-like and effector-like subsets, and the other represented long-term (LT) stem-like T cells. By ATACseq, LT stem-like CAR T cells had lower chromatin accessibility at genes associated with effector and exhaustion differentiation and higher BACH2 activity than the transitional cells.
When CD62L+ LT stem-like and CD62L- subsets from pre-infusion anti-CD19 CD8+ CAR-T were transferred into mice bearing E2A-PBX1 pre-B-cell acute lymphocytic leukemia (ALL), the CD62L+ subset expanded more, and there was a higher percentage of CD62L+CX3CR1- stem-like cells among their progeny.
In this model, mice clear leukemia within 7 days of CAR-T infusion. CAR-T cells obtained 20 days after infusion maintained an LT stem-like cluster with Bach2 expression and downregulated Tox. To assess their proliferative and differentiating potential, Tcf7-CD8+ and Tcf7+CD8+ CAR-T cells from Tcf7-GFP reporter mice were obtained 7 days after infusion into leukemia-bearing mice and were transferred into a new cohort of leukemia-bearing mice. In this second cohort, the Tcf7- cells induced more terminally differentiated (PD-1+TIM3+) progeny. However, when CD62L+Tcf7+CD8+ LT stem-like CAR-T were re-transferred, the cells expanded more and had enhanced ability to retain the stem-like phenotype.
To determine how Bach2 expression modulation impacted the CAR-T function, the researchers created a BACH2 knockout of the CAR-T cells. This resulted in a reduction in the number of CAR-T cells in the spleen 7 days after transfusion, a reduction in the frequency of TCF1+ and CD62L+CD8+ CAR-T, and upregulation of immune checkpoints, suggestive of fewer stem-like cells. Re-transferring these cells into new mice with leukemia revealed an impaired recall response, fewer stem-like cells, and increased PD-1 and TIM3 expression. These data suggested that BACH2 expression was necessary in CAR-T for their LT stem-like transcriptional program and antitumor responses.
To fine-tune BACH2 expression, BACH2 was fused with a destabilizing domain (DD) that would result in its degradation of BACH2, unless the domain was stabilized by Shield-1 treatment, and varying the dose of Shield-1 achieved various expression levels. In an in vitro T cell exhaustion model with persistent tonic signaling of GD2-targeted CAR-T, BACH2OE increased the frequency of the CD62L+ stem-like subset and downregulated expression of immune checkpoints. Cells treated with modulated BACH2 expression also had higher frequencies of the CD62L+ stem-like subset and lower levels of PD-1 than control CAR-T. Downregulation of TIM3 occurred in a dose-dependent manner with increasing BACH2 expression.
Finally, the researchers determined whether BACH2 induction during manufacturing could improve stemness and antitumor effects of the CAR-T in vivo. BACH2 was fused to ERT2 to allow tamoxifen to control the nuclear translocation of the fusion protein in vitro. Nuclear translocation increased the frequency of the CD62L+ stem-like subset and resulted in a reduction in TIM3 and PD-1 expression. Treatment of mice bearing neuroblastoma with these BACH2-programmed CAR-T resulted in better tumor control than controls.
The third paper examined intrinsic signaling in the absence of antigen, i.e. “tonic signaling”, in CAR-T. Tonic signaling has detrimental effects in CARs with a CD28 costimulatory domain, whereas it does not affect the function of CARs with a 4-1BB domain. Using a controlled system for tonic signaling, Chang et al. began by demonstrating that tonic signaling in 4-1BB-containing CAR-T results in increased BACH2 expression.
To uncover the mechanism, Chang et al. manufactured CD22-targeted CD28-containing CAR-T cells to overexpress BACH2, which resulted in lower PD-1, LAG3, and TIM3 expression at the end of manufacturing, and the cells acquired a more 4-1BB-like phenotype. BACH2OE in the CD28 CAR-T significantly increased Tcm and reduced Tem populations; there were more naive and memory lineage cells, and cells maintained cytotoxic function. This resulted in tumor control in vivo similar to that of the 4-1BB version. When BACH2 was disrupted in the 4-1BB cells, the opposite effect was observed.
To determine whether overexpression of BACH2 extended CAR-T cell function in the long term in response to chronic antigen exposure, cells were subjected to re-stimulation assays. CD28 BACH2OE CAR-T cells showed an initial increase in proliferation, followed by a rapid contraction. The Tcm phenotypes were maintained over time, and cells did not express high levels of CD39 or PD-1, suggesting exhaustion did not explain T cell failure.
The researchers hypothesized that this lineage restriction might be due to the maintained high BACH2 expression during chronic antigen exposure. To test this, they used a trimethoprim-controlled DD to titrate BACH2 expression. Interestingly, low levels of BACH2 from leakiness of the DD system resulted in enhanced and sustained T cell expansion and a greater transition from Tcm to Tem cells than under higher-expression conditions, consistent with the effect of fine-tuning observed by Conti, Evans, von Linde, Deguit, et al.. In a human ALL xenograft model, lower BACH2 levels in the CAR-T delayed tumor growth, improved survival, and were associated with greater CAR-T expansion and persistence.
Tonic signaling in high-affinity CAR-T targeting GD2 has been shown to drive the rapid onset of T cell exhaustion. To overcome this, BACH2 expression was induced to determine whether it could alter the developmental trajectory of T cells expressing this highly toxic CAR. BACH2 decreased the expression of TIM3 and LAG3 during manufacturing and improved T cell function when chronically exposed to antigen-expressing cells. The low-expression cells transitioned from Tcm to Tem; however, this transition did not prevent terminal differentiation or the expression of CD39 and PD-1 in this model, in contrast to the CD22 model. Enhanced BACH2 expression in CAR-T cells significantly improved tumor control. Unexpectedly, after two weeks, the mice experienced severe weight loss, which is under investigation.
Finally, both CAR-T studies analyzed samples of human CAR-T cells used in therapy to determine how BACH2 affected treatment outcomes. Hu, Zhu, Luo, et al. showed that anti-CD19 CAR-T infusion products from patients with relapsed or refractory (R/R) B cell lymphoma contained a subset of BACH2-expressing LT stem-like cells. As these cells differentiated into effector-like subsets, BACH2 expression decreased. Patients achieving complete remission (CR) with this treatment showed upregulation of LT stem-like signature genes, including BACH2, in their CD8+ CAR T cells. Similarly, Chang et al. found that the vast majority of anti-CD19 4-1BB-based CAR T cells developed for children with R/R ALL were of a Tcm or Tem phenotype, with higher BACH2 expression in the Tcm cells. Patients with sustained CR had CAR-T products with higher BACH2 scores, and those with higher BACH2 expression were more likely to remain in remission.
Overall, these three studies show the importance of BACH2 expression in regulating T cell differentiation, particularly the transition from the long-term, stem-like phenotype. If the suggested fine-tuning of BACH2 expression could be used during the manufacturing of patient T cell products for cancer therapy to optimize T cell phenotype and fate, it may improve their efficacy in solid tumors.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author on “Fine-tuning BACH2 dosage balances stemness and effector function to enhance antitumor T cell therapy” Alberto G. Conti answered our questions.
What was the most surprising finding of this study for you?
AGC: What surprised us most was the non-linear relationship between BACH2 dosage and T cell behavior. Having studied BACH2 overexpression extensively, we initially expected lower doses to simply produce a "diluted" version of the high-dose phenotype. Instead, we observed a completely distinct biological state. Certain genes were uniquely regulated only at low expression levels, while others remained constant regardless of dosage. It was as if the cells were interpreting the same transcription factor through a completely different lens. This underscores the vast "hidden" biology residing in even well-studied factors when we look beyond binary on/off states.
What is the outlook?
AGC: The next logical step is clinical translation. While there is immense interest in therapeutic T cells with a stem-like phenotype, progress has been limited by our understanding of how to precisely control memory transcription factors. This work provides a possible framework for balancing stemness with effector function through dosage control. We are now exploring how broadly these principles apply to other factors, and how to optimize these insights for next-generation therapies. We are actively pursuing this work both in our academic lab and through a spin-out company to bring these findings to patients.
If you could go back in time and give your early-career self one piece of advice, what would it be?
AGC: I would advise my younger self to spend more time thinking about which problems are worth solving, and to tackle the "riskiest" part of a project first. In our lab, we call this the "last experiment first” mentality. It’s tempting to start with “easy” or less risky aspects of a project, but addressing the most challenging, make-or-break questions early on saves years of potential frustration and lets you focus on ideas that really matter or that are likely to work out productively. Biology is notoriously difficult to predict; establishing a framework that allows you to fail fast or pivot quickly is the most efficient way to achieve breakthroughs. Obviously for this to be effective, the models used need to be in place and robust enough to give you trustworthy answers, but adopting this mindset has not only accelerated our current projects, it has fundamentally changed how I approach new challenges.




