Immune checkpoint blockade (ICB) has become a staple in the treatment of numerous cancers, but its efficacy could still be improved. Recently, three separate clinical trials explored strategies to improve ICB in the clinic. In one study, Duttagupta, Messaoudene, et al. investigated fecal microbiome transplant (FMT) in combination with ICB in patients with melanoma or NSCLC. In a similar study, Porcari et al. tested FMT plus standard-of-care pembrolizumab (anti-PD-1) and axitinib (VEGFR TKI) in the setting of metastatic renal cell carcinoma (mRCC). Lastly, Kendra et al. tested the use of anti-PD-1 as a neoadjuvant therapy in patients with resectable desmoplastic melanoma. The results of the two FMT trials were recently published in Nature Medicine, while the neoadjuvant trial was published in Nature Cancer.
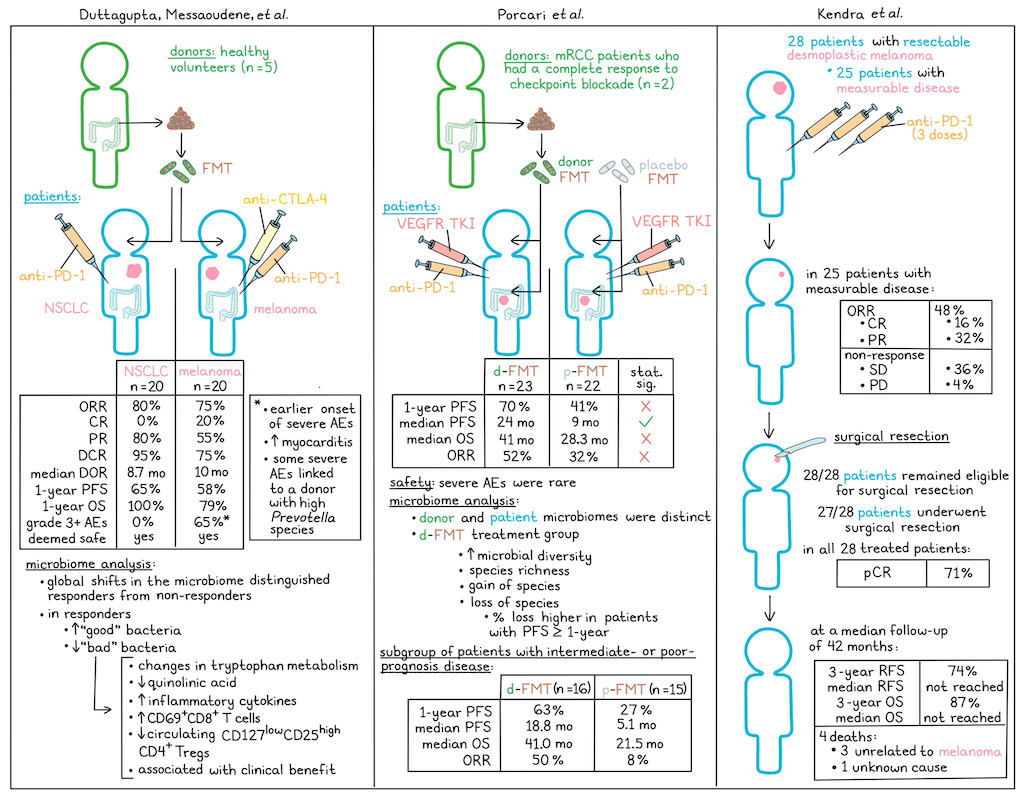
Following promising results in their phase 1 MIMIC trial, Duttagupta, Messaoudene et al. initiated a phase 2 clinical trial assessing healthy donor FMT by oral capsules prior to anti-PD-1 in non-small cell lung cancer (NSCLC; n=20) or to anti-PD-1 plus anti-CTLA-4 in melanoma (n=20) as first-line treatments. The overall response rate (ORR) in the NSCLC cohort was 80% (all partial responses), exceeding the prespecified primary endpoint of 64%. Further, the disease control rate (DCR) was 95%, as 3 of the 4 non-responders did experience stable disease for over 6 months. The median duration of response (DOR) was 8.7 months. The progression-free survival (PFS) and overall survival (OS) rates at 1 year were 65% and 100%, respectively. In the melanoma cohort, the ORR was 75% (4 complete responses and 11 partial responses), as was the DCR. The median DOR in this cohort was 10 months, and the PFS and OS at 1 year were 58% and 79%, respectively.
Evaluating safety, the researchers observed no grade 3 or higher adverse events (AEs) in NSCLC. While 65% patients experienced grade 3 or higher AEs in melanoma, they were consistent with those previously observed with anti-PD-1 + anti-CTLA-4. However, severe AEs did seem to appear earlier on in treatment, and cases of myocarditis were higher than in similar trials, warranting further monitoring in the future. Ultimately, the combination of FMT with the treatment in both cohorts was deemed safe by an independent data safety and monitoring committee.
Interestingly, 6 of the 13 patients who experienced grade 3 or higher AEs and 2 of the 3 patients who experienced myocarditis all were treated with samples from the same donor, and all of the samples from this donor given to patients in the melanoma cohort led to grade 3 or higher AEs. Further investigation revealed that this toxicity was likely linked to enrichment of Prevotella species.
Investigating possible connections between FMT donors and response, the researchers identified global shifts in microbiomes that distinguished responders from non-responders in both cohorts. In responders, previously identified beneficial bacteria were enriched, and deleterious bacteria were depleted, while in non-responders, deleterious bacteria were enriched after FMT. Further, the magnitude of species-level genome bin (SGB) loss calculated from patients at baseline was significantly higher in responders compared to non-responders after FMT, and the top SGBs lost in responders were taxa that have previously been associated with ICI resistance. Importantly, loss of baseline SGBs was more strongly associated with response than acquisition of donor-derived SGB, suggesting that a loss of “bad” bacteria had a stronger impact than a gain in “good” bacteria. This hypothesis was further validated in a mouse model.
Further investigation into the molecular mechanism underlying this the benefit of SGB loss revealed that patients with higher bacterial loss showed changes in the tryptophan metabolic pathway, reduced quinolinic acid, increased inflammatory cytokines, increased CD69+CD8+ T cells, and decreased circulating CD127lowCD25highCD4+ Tregs compared to patients with low bacterial loss. These results suggested that the elimination of baseline “bad” bacteria helps to alleviate an immunosuppressive metabolic and systemic immune milieu that would otherwise compromise ICI responses.
Overall, these results support the potential of FMT to safely overcome primary resistance to ICI in NSCLC and melanoma, and will be further assessed as part of the CanBiome2 trial evaluating FMT in combination with checkpoint blockade in 128 patients.
In a similar study, Porcari et al. evaluated 45 patients in the investigator-initiated, randomized, double-blind placebo-controlled phase 2a TACITO trial, in which patients with metastatic renal cell carcinoma (mRCC) were treated with either a placebo (p-FMT) or donor (d-FMT) FMT in combination with standard-of-care pembrolizumab (anti-PD-1) plus axitinib (VEGFR TKI) as a first-line treatment. Donor FMT samples came from RCC patients who had completely responded to either nivolumab or nivolumab + ipilimumab immunotherapy. The first FMT was done by colonoscopy, while two subsequent FMT treatments were given through oral capsules over 6 months. The primary endpoint of PFS at 12 months was not met (70% for d-FMT vs 41% for p-FMT), but the secondary endpoint of median PFS was met, with a median PFS of 24 months in the d-FMT arm vs. 9 months in the p-FMT arm. OS was also longer in the d-FMT arm (41.0 months) than in the p-FMT arm (28.3 months), but not significantly. Similarly, the ORR was 52% in the d-FMT arm and 32% in the placebo arm, but this difference was not significant.
In this trial, AEs related to the FMT were rare, with one grade 2 AE, one grade 3 AE, no treatment-related deaths, and no transmission of any infectious agents. The most common AEs and most common severe AEs included diarrhea and aspartate aminotransferase/alanine aminotransferase (AST/ALT) increase, which occurred similarly in both cohorts.
Evaluating the effects of FMT, the researchers found that while both donor and patient microbiomes had similar levels of diversity, they had unique microbial compositions, with specific species that were abundant in donors and rare in patients, or rare in donors but abundant in patients. After treatment, patients in the d-FMT arm showed increased microbial diversity and species richness, with notable increases and losses of particular species. While most of these individual factors could be linked to outcomes, the percentage of baseline species lost at follow-up was found to be higher among individuals with PFS>12 months in the d-FMT arm, but not the pFMT arm, suggesting that species loss may be linked to favorable clinical responses.
Finally, the researchers evaluated efficacy outcomes in a sub-group of patients with intermediate-prognosis or poor-prognosis disease based on IMDC classification, and found that in this subpopulation (d-FMT n=16; pFMT n=15), the differences in efficacy outcomes between the two treatment groups became much more pronounced. Here, the 12-month PFS was 63% vs. 27%, median PFS was 18.8 months vs. 5.1 months, median OS was 41.0 months vs. 21.5 months, and the ORR was 50% vs. 8% in the d-FMT vs. pFMT arms, respectively. Overall, these findings support the safety and potential efficacy of selected donor FMT to enhance ICI-based treatment in mRCC, with benefits that were particularly evident in patients with intermediate or worse prognosis.
In a third clinical trial, Kendra et al. took a different approach to improving immunotherapy by treating patients with resectable desmoplastic melanoma with neoadjuvant therapy to potentially reduce the need for radical surgery and radiation therapy. In the first cohort of the phase 2 SWOG S1512 trial, 28 patients were treated with pembrolizumab every 3 weeks for 3 doses prior to surgical excision. The primary endpoint of pathological complete response (pCR) was met at 71%, based on analysis of patient samples taken before treatment, at 3-5 weeks after treatment initiation, and at the time of surgery. Among the 25 patients with measurable disease at baseline, 16% had a complete response (CR) and 32% had a partial response (PR), for an ORR of 48%. Stable disease was observed in 36% of patients, while 4% showed disease progression. Further, of the 28 patients who began neoadjuvant pembrolizumab, all remained eligible for surgical resection and 27 underwent surgical resection of their tumor. Patients with lymph node involvement at baseline also underwent lymphadenectomies. At a median follow-up of 42 months, the 3-year relapse-free survival rate (RFS) was 74% and the median RFS has not been reached. The 3-year OS rate was 87% and the median OS has not been reached. Among the 4 patient deaths, 3 were unrelated to melanoma and the 4th was of unknown cause.
Looking at safety, the researchers found that the most common treatment-related AEs were fatigue, maculopapular rash, and diarrhea. Two patients experienced grade 3 AEs that led to discontinuation of treatment; however, both patients were still able to undergo surgical resection as scheduled. Additionally, the researchers performed genomic analysis of tumors across time points and found that there was no significant difference in TMB between patients with and without pCR.
Overall, the results from this first study cohort showed that neoadjuvant pembrolizumab in patients with resectable desmoplastic melanoma results in a high pCR rate, strong long-term outcomes, and an acceptable safety profile.
Together, these three trials show the potential to improve immunotherapy and outcomes for patients with a variety of cancers.
Write-up and image by Lauren Hitchings




