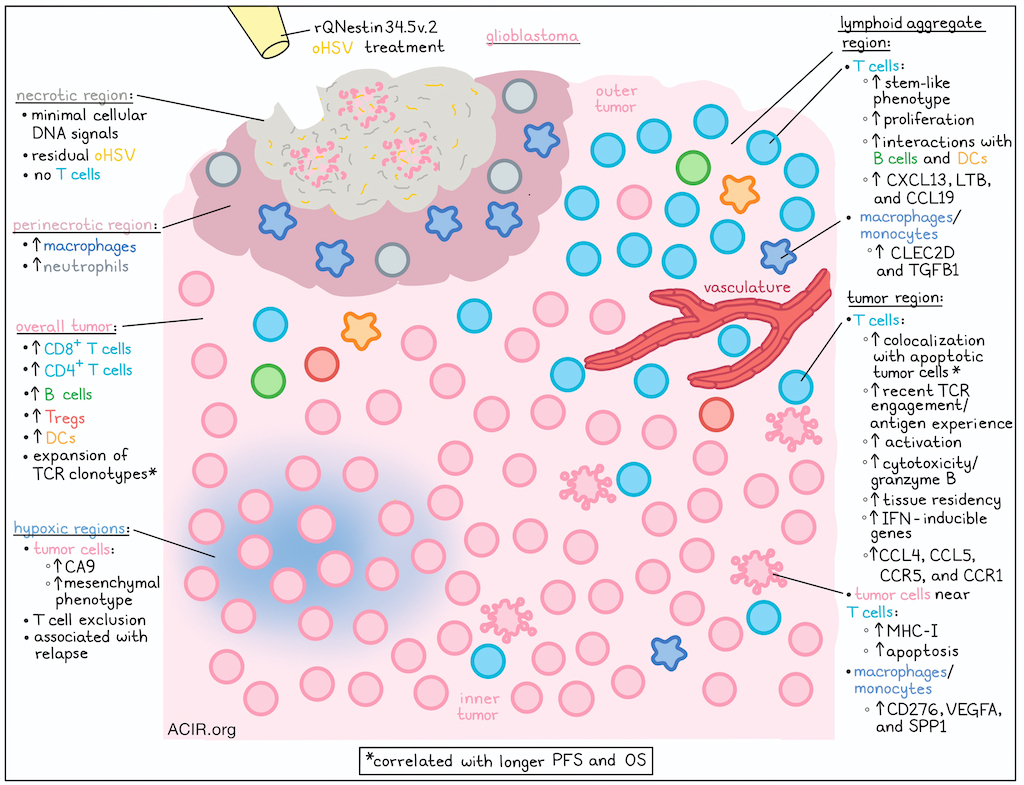
In a recent clinical trial, patients with glioblastoma (GBM) were treated with a single dose of an oHSV-1-based oncolytic viral therapy, rQNestin34.5v.2 (CAN-3110, linoserpaturev), in which expression of the viral ICP34.5 gene was controlled by the nestin promoter, overexpressed in GBM. Following up on evidence that treatment was associated with immune activation signatures, Meylen, Tiian, Wu, Ling, et al. utilized highly multiplexed spatial proteomics (CODEX) and spatial transcriptomics (Xenium) technologies to analyze tumor samples. They found that pre-existing TILs expanded upon treatment, resulting in deep infiltration and persistent T cell activation against tumor cells, which correlated with tumor cell apoptosis and improved progression-free and overall survival outcomes. Their results were recently published in Cell.
To begin, the researchers used CODEX and Xenium technologies along with TCR repertoire analysis to interrogate 28 large tumor regions from 16 post-treatment cases and 21 regions from 16 pre-treatment biopsies. Upon spatial mapping of cells, they found that oHSV treatment increased GBM infiltration by CD8+ T cells, CD4+ T cells, B cells, and Tregs, and increased the CD8+:Treg ratio. Further, T cells were found to deeply infiltrate post-treatment tumors, even at low treatment doses and long time-points after treatment.
Paired analysis of pre- and post-treatment samples from 8 patients identified expanded TCR clonotypes. In two representative patients, extensive and deep infiltration of both CD4+ and CD8+ T cells was observed, even at late time points, but infiltration varied by tumor region. In outer regions of the tumor or closer to the vasculature, T cells formed dense clusters of lymphoid aggregates, while deeper in tumors, T cells were more sparse, and more co-localized with tumor cells. Further, cytotoxic (granzyme Bhigh) CD8+ and CD4+ T cells were positively correlated with the density of apoptotic (cleaved caspase-3+) tumor cells, while granzyme Blow CD8+ T cells were negatively correlated with the density of apoptotic tumor cells across tumor regions. Similar gradients were observed using a nearest-neighbor analysis approach, with T cells closer to tumor cells showing high levels of granzyme B, PD-1, and CD44, indicative of antigen experience and cytotoxicity. Meanwhile, T cells further away and/or in lymphoid niches showed higher levels of Ki67, indicative of proliferation. Looking at tumor cells, those that were more distant from T cells were marked by CA9, indicative of more hypoxic cells, while tumor cells that were closer to T cells showed higher levels of MHC-I.
Investigating whether these patterns correlated with clinical outcomes, the researchers found that closer distances between GZMBhigh-med T cells and tumor cells was correlated with longer progression-free survival and lower tumor growth rates, consistent with T cell-mediated antitumor immunity. Longer distances, on the other hand, were correlated with shorter PFS and increased tumor growth following treatment with oHSV, consistent with a limited T cell response.
Next, Meylen, Tiian, Wu, Ling, et al. evaluated their data for a variety of T cell programs, including early activation, tissue residency, cytotoxicity, proliferation, stem-like state, interferon pathway, and exhaustion. CD8+ T cells showing evidence of recent TCR engagement (CD69 and NR4A1/NUR77) were spatially co-localized with tumor cells, and post-treatment samples showed a strong expansion of CD8+ tissue-resident T cells, as well as CD8+ stem-like T cells and CD4+ Tregs. T cells that were closer to tumor cells were more enriched for tissue residency, interferon-inducible genes, and expression of chemokines CCL4 and CCL5 and their corresponding receptors CCR5 and CCR1, consistent with activated T cells recruiting more T cells. Meanwhile, T cells farther from tumor cells were more enriched for stem-like features.
Within lymphoid aggregates, 35% of T cells were stem-like CD8+ cells, closely interacting with B and dendritic cells, the latter of which also showed enrichment after oHSV. In lymphoid aggregate “neighborhoods”, T cells upregulated lymphoid tissue organizing genes (CXCL13, LTB, and CCL19) and B cell/dendritic cell interaction markers, while T cells in tumor neighborhoods expressed interferon, tissue residency, and early activation markers. Looking at macrophages and monocytes, the researchers found that their expression of immunosuppressive molecules also varied depending on their neighborhood, with cells in lymphoid aggregate regions preferentially expressing CLEC2D and TGFB1, and cells in tumor cell regions exhibiting high levels of CD276, VEGFA, and SPP1.
Next, to determine whether T cells were reacting to any residual oHSV antigens, the researchers tracked residual HSV antigens by immunohistochemistry and found that they were only present in necrotic regions with little to no cellular DNA signal and essentially no T cells. However, HLA-DR+ macrophages and neutrophils were enriched at the borders surrounding necrotic areas (perinecrotic regions) with residual HSV antigen. Looking at bulk tumor and blood TCRβ chain (TRBC) sequencing from pre- and post-oHSV, the researchers noted significant expansion of T cell clonotypes and an increase in clonality in tumors, but not in matched PBMCs after oHSV treatment. Patients with post-treatment expansion had longer overall survival than those with contracted repertoires. T cells specific for HSV-1 or HSV-2 were not identified in patient samples, but a tumor-reactive signature was enriched in T cells proximal to tumor cells in all post-treatment samples, suggestive of epitope spreading. Expanded T cell clones also showed high levels of tissue residency and cytotoxic markers and were in close proximity to tumors, suggesting that tumor proximity may reflect TCR specificity. Further, treatment with dexamethasone, often used to control side effects associated with GBM, correlated with reduced T cell clonality following oHSV, suggesting it could impair antitumor immunity.
Finally, the researchers investigated samples from patients with less favorable clinical responses, in search of possible resistance mechanisms. Analysis of tumor samples showed that after oHSV treatment, tumor cell states were altered, with a marked expansion of tumor cells with a hypoxic mesenchymal signature at the time of relapse. VEGFA expression was found to be independently correlated with this tumor gene signature, and could be used to identify hypoxic regions, which excluded T cells, despite infiltration in adjacent non-hypoxic regions.
Overall, these results shed light on the induction of T cell-mediated antitumor immunity in GBM that has been treated with oncolytic viral therapy. The expansion of pre-existing T cell clones and the spatial relationships between cytotoxic T cells and apoptotic tumor cells may be clinically relevant as indicators of progression-free and overall survival outcomes. Further, these results illuminate tumor cell-mediated hypoxia as a possible resistance mechanism, and dexamethasone treatment as a possible antagonist to antitumor immunity. These new insights could help to guide future decision-making regarding effective clinical combinations with oHSV treatment for GBM.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, Maxime Meylan and Ye Tian, two co-first authors on this paper, answered our questions.
What was the most surprising finding of this study for you?
The most surprising result of this study is that a single injection of an oncolytic virus (OV) could ignite a powerful and long-lasting T cell reaction, sometimes even years after treatment. This revolutionizes our understanding of the mechanism of action of oncolytic virotherapy, which was primarily designed to kill tumor cells. We were particularly stunned to directly see tissue-resident T cell clones expand and perform cytotoxic actions in situ, where granzyme B-positive T cells were positioned adjacent to apoptotic tumor cells. These observations highlight the durable immunological impact of the therapy and the power of spatial analysis in revealing functional immune–tumor interactions
What is the outlook?
This study opens the door for immunotherapy in glioblastoma. By driving a massive influx of T cells into a tumor that was long considered immunologically cold, OV treatment now enables the design of rational combinations such as anti-PD-1/VEGF bispecific antibodies that may improve T cell access to resistant hypoxic tumor regions identified through spatial analysis. In parallel, approaches targeting tissue-resident T cells – including those involving IL-15 or 4-1BBL – may further enhance durable antitumor immunity. Together, these directions highlight how spatially resolved insights can guide next-generation combination therapies.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
MM: As it becomes increasingly easy to generate massive amount of data, it is important more than ever to anchor that data in a concrete biological question. While sometimes we feel the need to layer sophisticated computational methods simply because they are available, this rarely brings actionable insights. The real breakthroughs often come from first distilling the fundamental question, and then taking the simplest, but rigorous approach that ultimately leads to strong and interpretable results.
YT: My advice would be: master the technology, but stay anchored in the biology. In the era of spatial omics and large-scale datasets, it is easy to become captivated by technical sophistication. Yet the most impactful discoveries begin with a clear biological or clinical question. Developing deep methodological expertise has been immensely rewarding for me, but its true value lies in enabling meaningful scientific insight rather than simply exploring new tools. Keeping that balance helps ensure that innovation serves understanding — not the other way around.




