Cancer immunology studies are often performed using mouse models or tumor samples from patients, but these models do not provide ideal opportunities to study immune cells as they interact with endogenous tumors. To visualize tumor–immune interactions in situ, Ludin and Stirtz et al. generated transgenic zebrafish models and adapted a water flow system to allow for live tracking of tumor and immune cells in endogenous melanoma. Using this novel transparent model system and multiple imaging and analysis tools, the researchers identified regions of tumors called CRATERs that appeared to play a unique role in responses to immunotherapy. The results of their studies were recently published in Cell.
To study tumor–immune interactions in situ in endogenous tumors, Ludin and Stirtz et al. generated transgenic zebrafish in which all T cells (marked by Ick expression) were fluorescently labeled with mCherry, and CD8α-expressing T cells were fluorescently labeled with EGFP to allow for tracking by in vivo imaging. In addition to labeling CD8+ T cells, EGFP also labeled a population of CD8α+ myeloid cells that were consistent with CD8+ DCs, found uniquely in mice
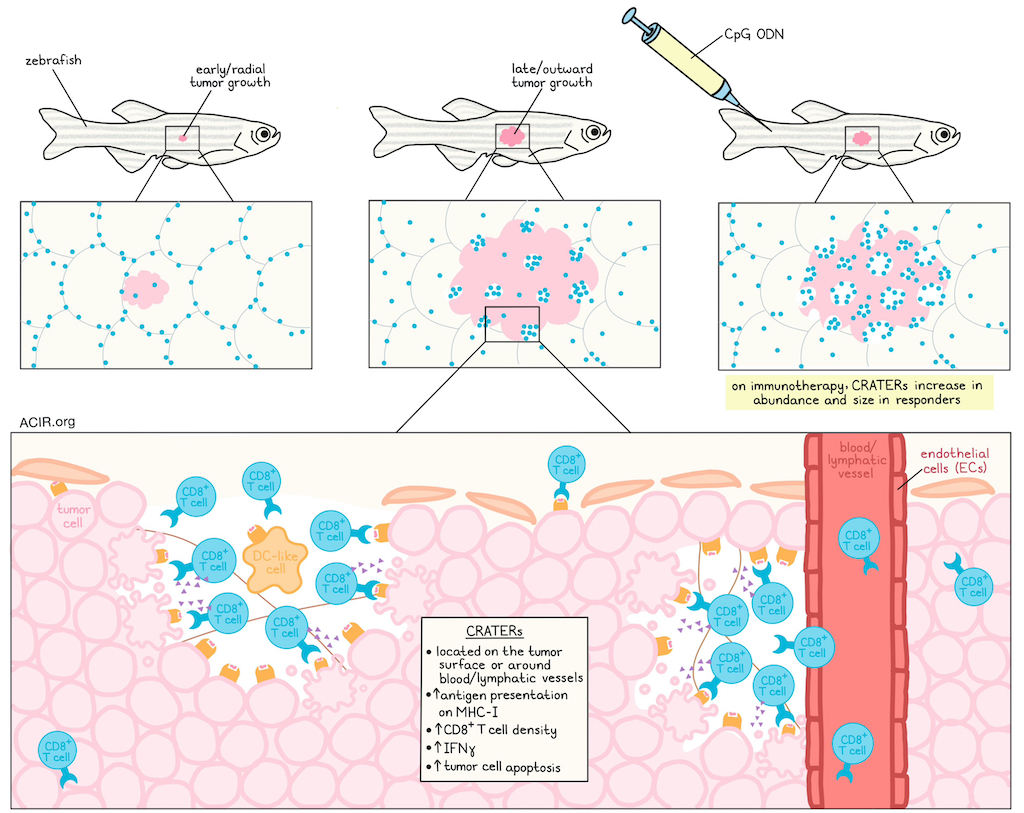
The researchers used longitudinal analysis to evaluate T cell infiltration into tumors over the course of endogenous tumor development (induced through one of two oncogene-inducing mechanisms) in the same fish. This revealed that in healthy fish skin and during early radial growth of pre-cancerous lesions, most T cells were CD8-, and were distributed along the edges of the fish scales, creating tesselated structures that served as sites of peripheral immune surveillance. However, as later-stage tumors grew outward, the tesselated pattern was disrupted, and CD8+ T cells infiltrated tumors, with enrichment in areas that were devoid of melanocytes.
Quantitative analysis of 3D confocal images confirmed that in all of the over 100 individual fish evaluated, CD8α+ cells preferentially localized to pockets lined by melanoma cells, termed termed cancer regions of antigen presentation and T cell engagement and retention (CRATERs), where the CD8+ T cell density was significantly higher than on the rest of the tumor surface. Time-lapse imaging in zebrafish tumors over the course of 15–20 hours showed that CD8+ T cells lingered in these CRATERs, interacting with melanoma cells for hours at a time, consistent with tumor recognition and target cell killing.
Most CRATERs were found to emerge in perivascular areas, adjacent to endothelial cell-lined blood and lymphatic vessels, and contained fine collagen fibers extending from the capsule surrounding the tumor. Potential DC-like cells were also identified in CRATERs.
Using high-resolution spatial transcriptomics on zebrafish tumors, the researchers found gene expression profiles that aligned with the structures and cell types they had identified in and around CRATERs. Differential gene expression analysis revealed that CRATER-related areas had elevated expression of b2m (encoding B2M, a component of MHC-I). When b2m was depleted specifically in tumors, the ratio of CD8+ T cells within CRATERs to those embedded in the tumor significantly decreased, and CD8+ T cells became more spread out across the tumor surface, suggesting that CRATERs are sites of tumor recognition.
Suspecting that CRATERs might facilitate CD8+ T cell interactions with tumor cells, the researchers treated zebrafish with CpG ODN – a TLR agonist that acts as an effective cancer immunotherapy in zebrafish. Following four daily injections of CpG ODN, CRATERs on tumors covered more of the surface and were larger than in control-treated or untreated tumors. These CRATERs maintained high CD8+ T cell densities and interactions with melanoma cells, dependent on B2M. The researchers also identified increased IFNγ+ CD8+ T cells and overall IFNγ levels in CRATERs following CpG ODN treatment, providing further evidence of immune responses at these sites.
To assess whether tumor cell killing occurs within CRATERs, the researchers established a model to measure apoptosis, and found that while minimal apoptosis was observed in CRATERs in PBS-treated WT or CpG ODN-treated B2M-KO tumors, CRATERs in CpG ODN-treated WT tumors showed increased apoptosis. CD8+ T cells were found in direct contact with apoptotic melanoma cells, suggesting T cell-mediated cytotoxicity, which could play a role in sculpting the CRATER architecture. Consistent with this, CRATER coverage was found to be lower (though B2M expression remained the same) in zebrafish models deficient in either T cells and B cells or T cells and NK cells. TGFβ inhibition for 24 hours had a similar effect to CpG ODN, leading to an accumulation of CD8+ cells in CRATERs and long interactions with melanoma cells. In this immunotherapy setting, the researchers also observed evidence of antigen uptake and mobilization by possible DCs.
Moving to mouse models, Ludin and Stirtz et al. inoculated mice with OVA-expressing melanoma tumors and then adoptively transferred OVA-specific OT cells. OT cells induced focal attrition, similar to CRATERs, at the tumor surface, but CD8+ T cells localized to areas of B2M expression across the tumor, likely due to the strong presentation of OVA over the whole tumor surface.
Moving to human models, the researchers examined melanoma samples, including cutaneous and mucosal melanoma, as well as primary tumors and metastases, and found that all contained structures consistent with CRATERs. As in zebrafish, these CRATERs were located at tumor borders and perivascular regions, were densely packed with CD8+ T cells (including some expressing granzyme B), had increased expression of HLA-A (corresponding to MHC-I) on tumor cells lining the CRATERs, and only formed during the vertical outward growth phase (not the early radial growth phase) of primary melanoma. Interestingly, DCs within CRATERS also expressed increased HLADBP1 (MHC-II), melanoma antigen MART-1, and PD-L1 compared to elsewhere in tumors, suggesting possible contributions to T cell priming.
Finally, the researchers examined melanoma samples from patients treated with immunotherapy, and found that while pre-treatment samples did not predict response, biopsies from responders (SD, PR, or CR) had higher linear densities of CRATERs, while non-responders (PD) had fewer CRATERs, with CD8+ T cells more dispersed throughout the tumors. A biopsy taken after a neoadjuvant ICB treatment had the highest linear density of CRATERs, suggesting that CRATERs may be even more extensive in samples that are not already demonstrating some level of resistance. In samples from patients treated with T-VEC, increased CRATERs, larger CRATERs, and increased accumulation of lymphocytes were also observed. Further, CRATERs were evident in samples of large-volume NSCLC tumors, suggesting that these structures may be relevant in other tumor types.
Overall, these results suggest that CRATERs, discovered in zebrafish models and observed in murine models and human tumor samples, are likely sites of antitumor immune activity in endogenous tumors where T cells recognize tumor antigens and kill target cells. Further, increased CRATER abundance could be used as an on-treatment prognostic indicator of immunotherapy response, as it demonstrates evidence of antitumor immunity.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, co-first author Aya Ludin answered our questions.

What was the most surprising finding of this study for you?
In this study, we found focal niches rich with antigen-presenting molecules on the surface of tumors – CRATERs. In CRATERs, CD8+ T cells recognized tumor cells and kill them. If we were to examine tumors using still or short time-lapse imaging, we would have concluded that CD8+ T cells resided in CRATERs. But we followed CD8+ T cell behavior in the tumor for 15-20 hours, and saw that CD8+ T cells aren’t “stuck” in those pockets. Rather, they present a behavior characteristic of antigen recognition – forming lengthy interactions with tumor cells and then moving on. This paved the way for our understanding of the true nature of the CRATERs.
What is the outlook?
CRATERs can be identified in patients’ biopsies upon pathological examination and strongly correlate with clinical benefit from immunotherapy. This presents two principal avenues to enhance immunotherapy efficacy. First, quantifying CRATER density along the tumor borders can serve as an early biomarker indicating treatment success. This can inform a clinician if an applied treatment is beneficial for their patient in real time. Second, as CRATERs promote tumor recognition and killing, activating pathways that lead to CRATER expansion could be used to boost immunotherapy efficacy.
What was the coolest thing you’ve learned (about) recently outside of work?
AL: I’ve learned that one needs to mature a bit to read Virginia Woolf, but it’s worth it. Here is what she wrote about great fiction: “For masterpieces are not single and solitary births; they are the outcome of many years of thinking in common…so that the experience of the mass is behind the single voice.” (Virginia Woolf, “A Room of One’s Own”)




